Sample Research Protocol Office Of Human Research
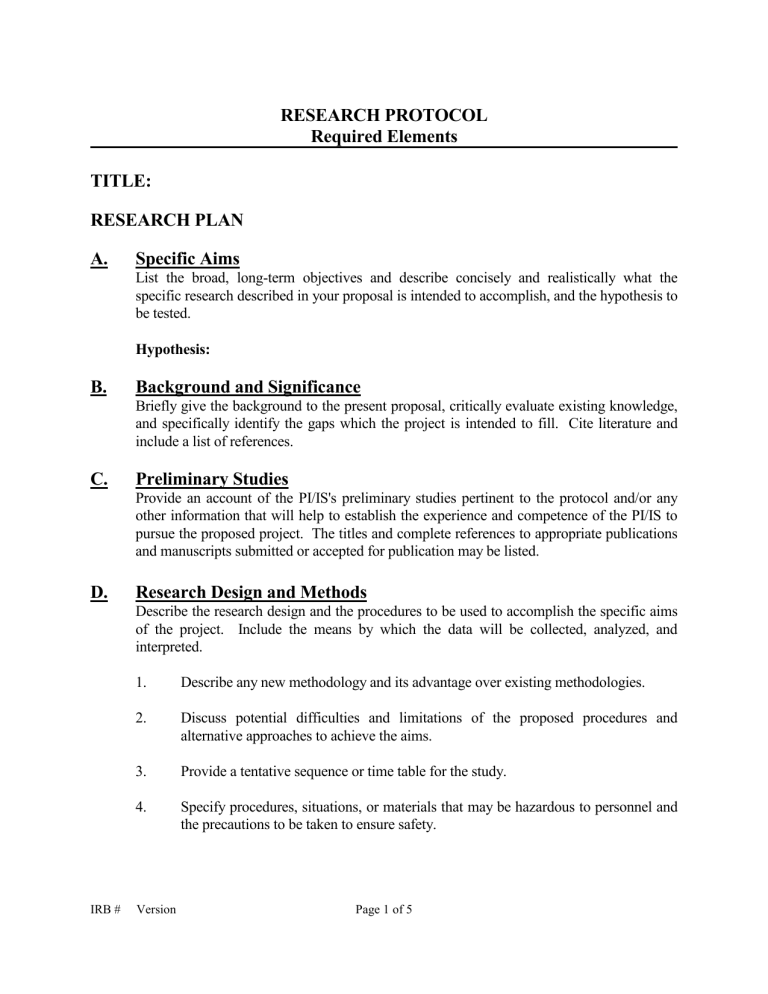
Sample Research Protocol Office Of Human Research A complete description of the protocol must be submitted with initial applications for irb or exempt review. the research protocol should provide the information needed for reviewers to determine that the regulatory and human research protection program (hrpp) policy requirements have been met. there is no required format or template; different. This protocol template aims to facilitate the development of two types of clinical trials involving human participants. the first type of trials are phase 2 and 3 clinical trial protocols that require a food and drug administration (fda) investigational new drug (ind) or investigational device exemption (ide) application. ind ide protocol word.

Qualitative Research Protocol Human Subjects Research The irb office has developed protocol templates for use by the northwestern university research community to describe research human research activities. consult our protocol conversion guide 11 9 2014 for important information about using templates with new submissions or converting templates for previously approved research. Human subjects office irb hardin library, suite 105a 600 newton rd iowa city, ia 52242 1098. voice: 319 335 6564 fax: 319 335 7310. 1. identify sources of research material obtained from individually identifiable living human subjects in the form of specimens, records or data. indicate whether the material or data will be obtained specifically for research purposes or whether use will be made of existing specimens, records or data. subjects with specific diseases or. Open in a separate window. first section: description of the core center, contacts of the investigator s, quantification of the involved centers. a research protocol must start from the definition of the coordinator of the whole study: all the details of the main investigator must be reported in the first paragraph.

Research Protocol Template 1. identify sources of research material obtained from individually identifiable living human subjects in the form of specimens, records or data. indicate whether the material or data will be obtained specifically for research purposes or whether use will be made of existing specimens, records or data. subjects with specific diseases or. Open in a separate window. first section: description of the core center, contacts of the investigator s, quantification of the involved centers. a research protocol must start from the definition of the coordinator of the whole study: all the details of the main investigator must be reported in the first paragraph. The office for human research protections (ohrp) provides leadership in the protection of the rights, welfare, and wellbeing of human subjects involved in research conducted or supported by the u.s. department of health and human services (hhs). ohrp is part of the office of the assistant secretary for health in the office of the secretary of. Abstract. background: institutional review boards (irbs), duly constituted under the office of human research protection, have the federally mandated responsibility of reviewing research involving human subjects to ensure that a proposed protocol meets the appropriate ethical guidelines before subjects may be enrolled in any study.

Ppt How To Write A Research Protocol Powerpoint Presentation Free The office for human research protections (ohrp) provides leadership in the protection of the rights, welfare, and wellbeing of human subjects involved in research conducted or supported by the u.s. department of health and human services (hhs). ohrp is part of the office of the assistant secretary for health in the office of the secretary of. Abstract. background: institutional review boards (irbs), duly constituted under the office of human research protection, have the federally mandated responsibility of reviewing research involving human subjects to ensure that a proposed protocol meets the appropriate ethical guidelines before subjects may be enrolled in any study.

Hrp 503 Human Research Protocol And Instructions Clean

Comments are closed.