1 Electron Configuration

List Of Electron Configurations Of Elements September 1, 2024 by jay. electron configuration chart of all elements is mentioned in the table below. the shorthand electron configuration (or noble gas configuration) as well as full electron configuration is also mentioned in the table. atomic no. Electron configuration. in atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] for example, the electron configuration of the neon atom is 1s2 2s2 2p6, meaning that the 1s, 2s, and 2p subshells are occupied by.
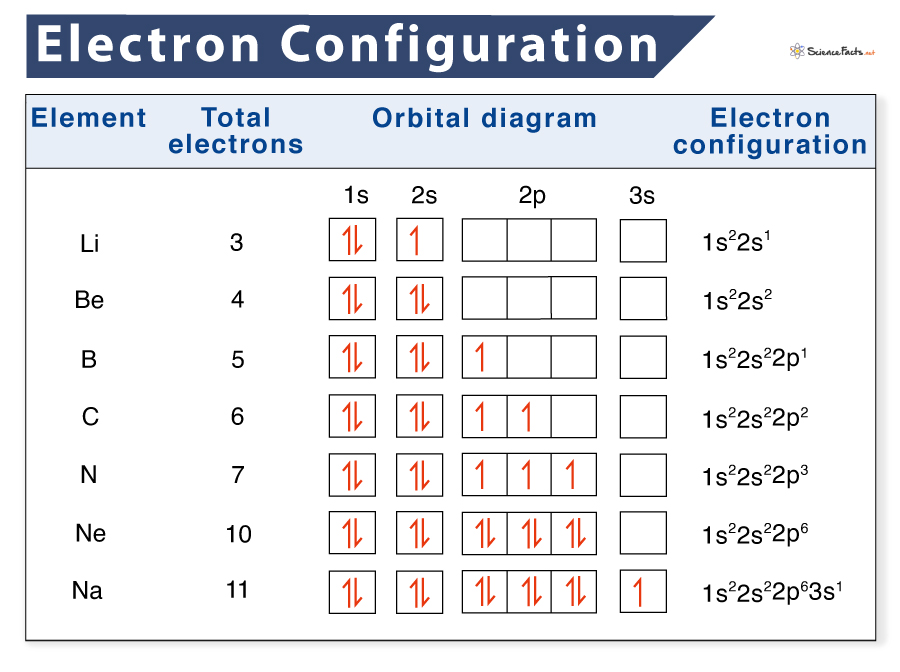
Electron Configuration Definition Examples Chart And Diagram In this case, 2 2 6 2 6 2 10 6 2 1= 39 and z=39, so the answer is correct. a slightly more complicated example is the electron configuration of bismuth (symbolized bi, with z = 83). the periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3. Otherwise, write the order of the energy levels with electron configuration chart: 1 s 2 2 s 2 2 p 3 \rm 1s^22s^22p^3 1 s 2 2 s 2 2 p 3. put the brackets around the electron configuration of the last noble gas, group 18 element, before nitrogen. note that helium (he) is the noble gas preceding nitrogen. continue with the electron configuration. Updated on february 01, 2021. the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. this handy chart compiles the electron configurations of the elements up through number 104. The electron configuration of argon is 1s 2 2s 2 2p 6 3s 2 3p 6, and thus the abbreviated notation of potassium is written as [ar] 4s 1. similarly, the electronic configuration of argon is written using its nearest inert neighbor neon, having the electronic configuration 1s 2 2s 2 2p 6 .

Electronic Structure Of Atoms Electron Configurations Chemistry Updated on february 01, 2021. the electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. this handy chart compiles the electron configurations of the elements up through number 104. The electron configuration of argon is 1s 2 2s 2 2p 6 3s 2 3p 6, and thus the abbreviated notation of potassium is written as [ar] 4s 1. similarly, the electronic configuration of argon is written using its nearest inert neighbor neon, having the electronic configuration 1s 2 2s 2 2p 6 . The electron configuration and the orbital diagram are: following hydrogen is the noble gas helium, which has an atomic number of 2. the helium atom contains two protons and two electrons. the first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, ml = 0, m s = 1 2). For hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (figure \(\pageindex{1}\)), and the electron configuration is written as 1s 1 and read as “one s one.” a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons.
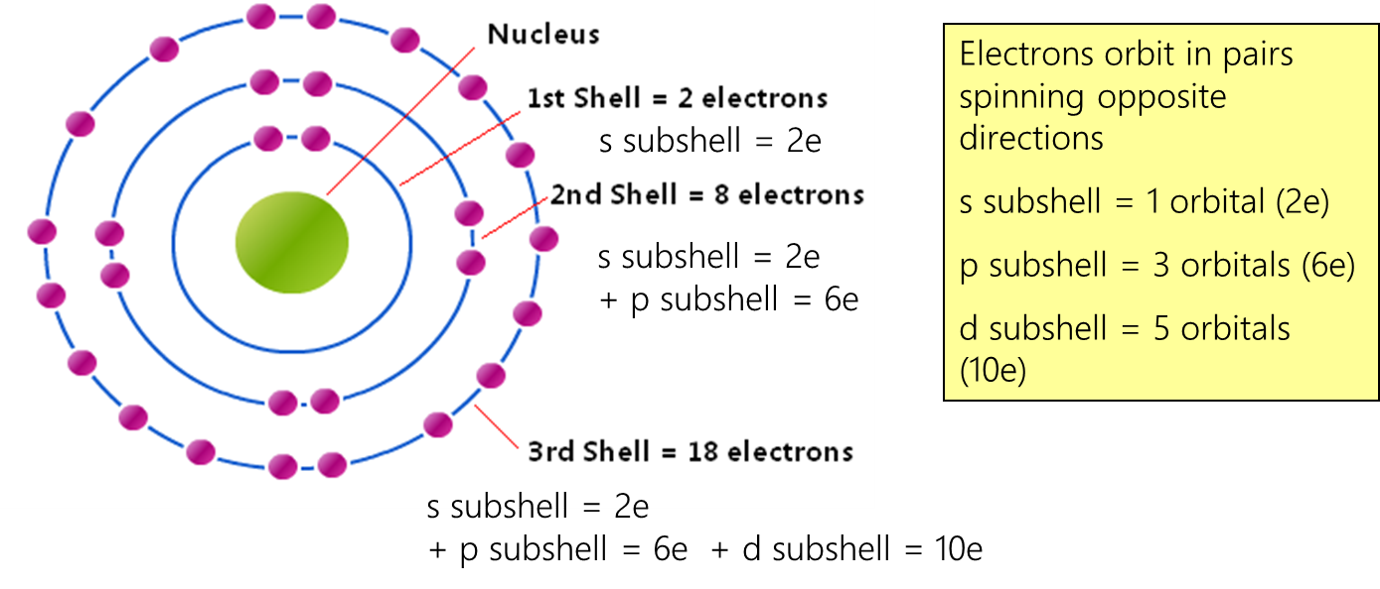
1 Electron Configuration The electron configuration and the orbital diagram are: following hydrogen is the noble gas helium, which has an atomic number of 2. the helium atom contains two protons and two electrons. the first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, ml = 0, m s = 1 2). For hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (figure \(\pageindex{1}\)), and the electron configuration is written as 1s 1 and read as “one s one.” a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons.
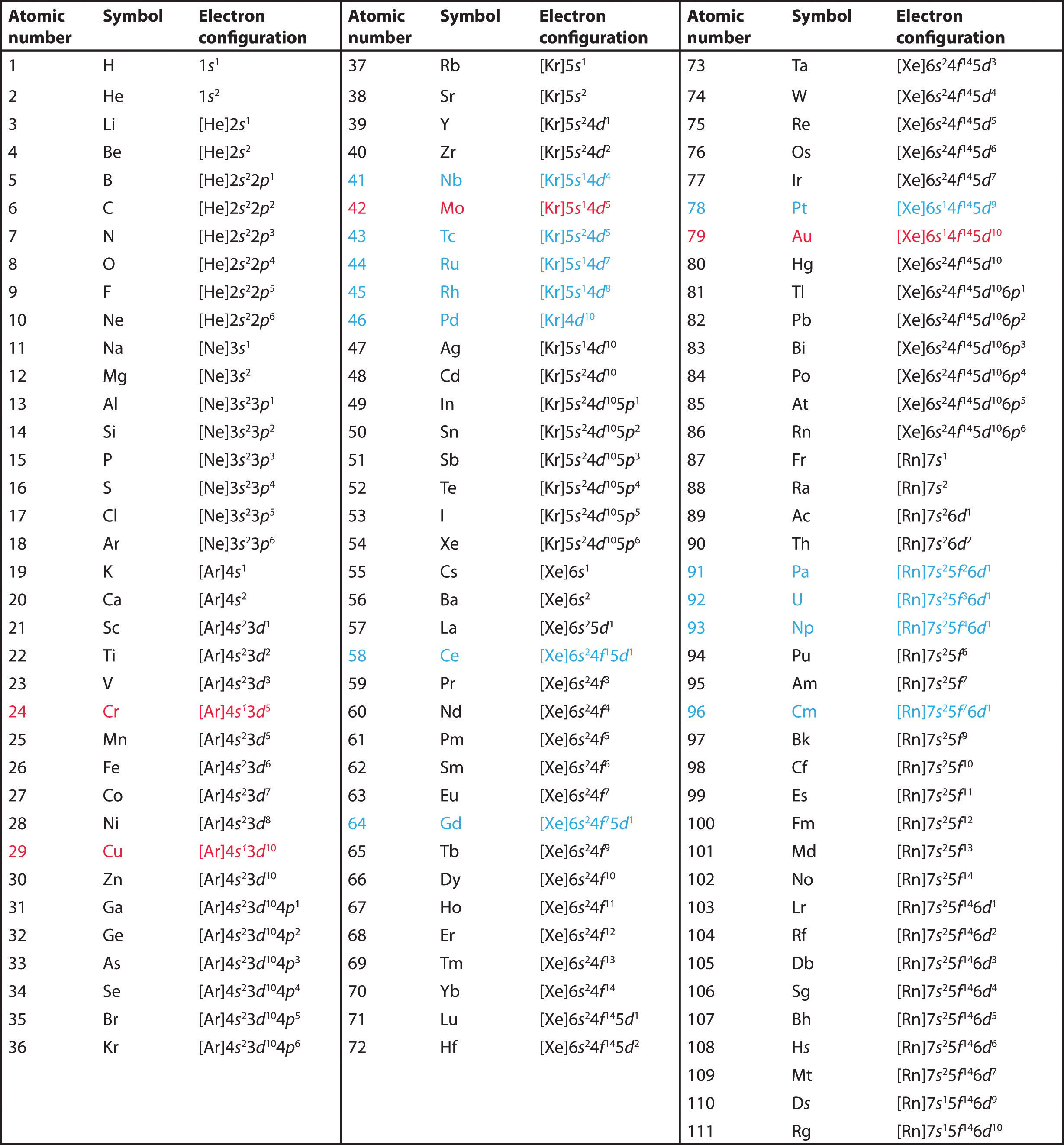
7 8b Electron Configurations And The Periodic Table Chemistry Libretexts

Comments are closed.