1 Synthesis Of Aspirin Experiment Chemistry Libretexts
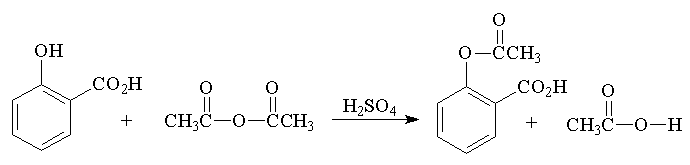
1 Synthesis Of Aspirin Experiment Chemistry Libretexts University of missouri–st. louis; chemistry) 1: synthesis of aspirin (experiment) is shared under a license and was authored, remixed, and or curated by libretexts. analgesics are compounds used to reduce pain, antipyretics are compounds used to reduce fever. one popular drug that does both is aspirin. Experiment 614: synthesis of aspirin . section 1: purpose and summary . conduct a chemical reaction to produce aspirin. separate the aspirin from the reaction by products using vacuum filtration. analyze the aspirin and estimate its purity. acetylsalicylic acid, commonly known as aspirin, is the most widely used drug in the world today.

Solution Synthesis Of Aspirin Experiment Studypool Synthesis of aspirin (acetylsalicylic acid) weigh out about 1 gram of salicylic acid on a piece of weighing paper. to do this, first weigh a piece of weighing paper. place some salicylic acid on the weighing paper and weigh again. add or remove solid until you have about 1 gram of it on the paper. The experiment was performed in the lab as follows: esterification reaction. add 138mg of salicylic acid, a boiling stone, and a drop of sulfuric acid to a conical vial, followed by .3ml of acetic anhydride. mix and take a tlc with 1:1 tlc solvent and note the rf. heat the mixture over a 90 degree celsius steam bath for 10 minutes. An experiment is described that is suitable for the early portion of the laboratory in a general chemistry course and integrates organic examples. it is the two step synthesis of aspirin starting from oil of wintergreen. the mechanism for this synthesis provides examples of three major classes of chemical reactions: hydrolysis, condensation, and proton transfer. to understand the chemistry. Acetylsalicylic acid is marketed under the name of aspirin for the home bayer being one of the drugs most consumed in the world. it was synthesized at the end of the last century by the german chemist felix hofmann. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic.
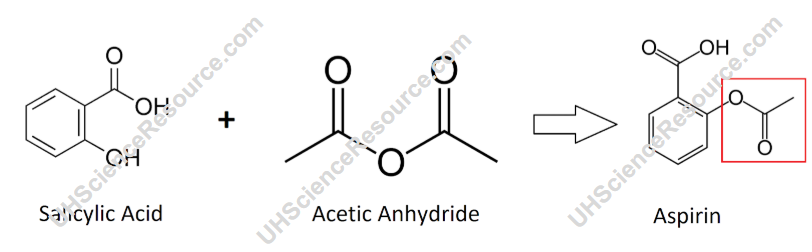
Synthesis Of Aspirin From Salicylic Acid An experiment is described that is suitable for the early portion of the laboratory in a general chemistry course and integrates organic examples. it is the two step synthesis of aspirin starting from oil of wintergreen. the mechanism for this synthesis provides examples of three major classes of chemical reactions: hydrolysis, condensation, and proton transfer. to understand the chemistry. Acetylsalicylic acid is marketed under the name of aspirin for the home bayer being one of the drugs most consumed in the world. it was synthesized at the end of the last century by the german chemist felix hofmann. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic. Aspirin screen experiment. experiments home. aspirin home. register. log in. in level 2, you'll learn about the chemical properties of aspirin. you'll get to purify your crude sample by recrystallisation, we recommend a starting weight of 5 g, and then analyse it using thin layer chromatography. in each activity you'll be able to collect points. Aspirin screen experiment. quickstart. log in. register. this resource has been developed in partnership with learning science and the university of bristol.

Chem O 16 Synthesis Of Aspirin 1 Pdf Experiment 16 The Synthesis And Aspirin screen experiment. experiments home. aspirin home. register. log in. in level 2, you'll learn about the chemical properties of aspirin. you'll get to purify your crude sample by recrystallisation, we recommend a starting weight of 5 g, and then analyse it using thin layer chromatography. in each activity you'll be able to collect points. Aspirin screen experiment. quickstart. log in. register. this resource has been developed in partnership with learning science and the university of bristol.

Experiment 1 Synthesis Of Aspirin Lab Pdf Kieran Russell Experiment

Comments are closed.