Acetylation Reaction With Acetic Anhydride

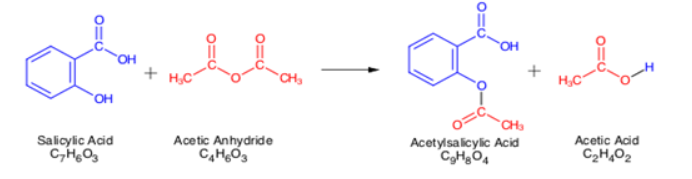
Acetylation Explain Acetylation Reaction Of Acetylation With An example of an acetylation reaction is illustrated below. reaction of acetylation of salicylic acid. here, salicylic acid is subjected to acetylation with the help of acetic anhydride to yield acetylsalicylic acid (commonly known as aspirin) and acetic acid as the final product. it can be noted that acetic anhydride is also used as an. Phosphomolybdic acid (pma) is a simple and efficient catalyst for the acetylation of structurally diverse alcohols, phenols, and amines. acetylation reactions with acetic anhydride proceed in excellent yield in the presence of a catalytic amount of pma at ambient temperature within a relatively short reaction time under solvent free conditions.
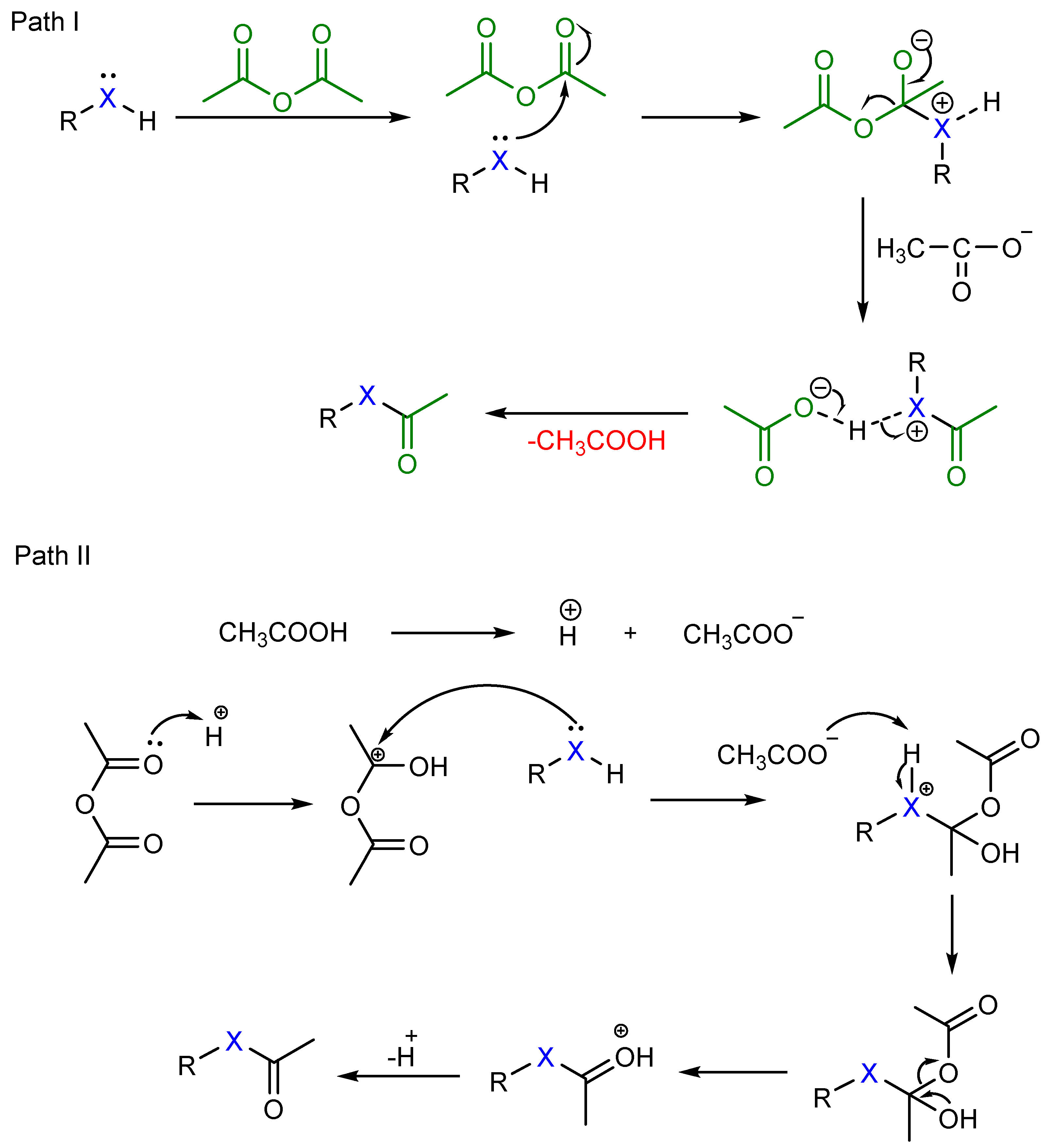
Acetic Anhydride Reaction Acetylation. in chemistry, acetylation is an organic esterification reaction with acetic acid. it introduces an acetyl group into a chemical compound. such compounds are termed acetate esters or simply acetates. deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Acetylation reactions are classically performed using excess of acetic anhydride (ac 2 o) in solvent free conditions or by eventually working with stoichiometric amounts of ac 2 o in organic solvents; both methods require the addition of basic or acid catalysts to promote the esterification. therefore, they usually lead to the generation of. Measure out 0.6 ml of acetic anhydride and prepare a solution of 530 mg of sodium acetate in 3 ml of water. add the acetic anhydride to the solution of aniline hydrochloride in water, mix by swirling, and immediately add the sodium acetate solution. the solution becomes white as acetanilide precipitates. cool the solution in an ice bath and. Acetylation reactions with acetic anhydride proceed in excellent yield in the presence of a catalytic amount of pma at ambient temperature within a relatively short reaction time under solvent free conditions. s. t. kadam, s. s. kim, synthesis, 2008, 267 268.
Acetylation Reaction Details With Mechanism Examples Measure out 0.6 ml of acetic anhydride and prepare a solution of 530 mg of sodium acetate in 3 ml of water. add the acetic anhydride to the solution of aniline hydrochloride in water, mix by swirling, and immediately add the sodium acetate solution. the solution becomes white as acetanilide precipitates. cool the solution in an ice bath and. Acetylation reactions with acetic anhydride proceed in excellent yield in the presence of a catalytic amount of pma at ambient temperature within a relatively short reaction time under solvent free conditions. s. t. kadam, s. s. kim, synthesis, 2008, 267 268. Protocol for o acetylation. a. dissolve the starting material (1.0 equiv.) in pyridine (2–10 ml mmol) under ar. b. add ac 2 o (1.5–2.0 equiv. for a hydroxy group) to the solution at 0°c. c. stir the reaction mixture at room temperature until the starting material is completely consumed as the progress of the reaction is monitored by tlc. d. Acetic anhydride is also commonly used to prepare n substituted acetamides from amines. for example, acetaminophen, a drug used in over the counter analgesics such as tylenol, is prepared by reaction of p hydroxyaniline with acetic anhydride.

Comments are closed.