Acid Base Equilibria Buffer Solution

Acid Base Equilibria Buffer Solution Youtube A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 7.1.1). Remember those pesky iceboxes? weak acids and bases establish equilibria, so we have to do iceboxes to figure out things about them. but don't worry, buffers.
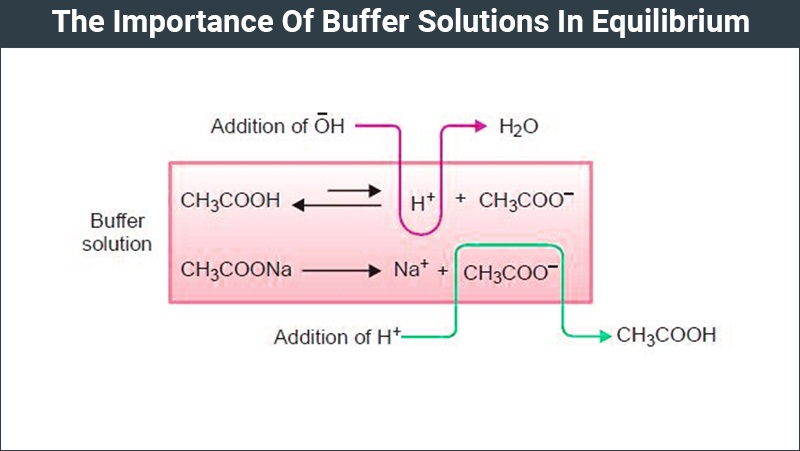
Buffer Solutions In Equilibrium Types Of Buffer Solution Chemistry A solution containing appreciable amounts of a weak conjugate acid base pair is called a buffer solution, or a buffer. buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure 7.7.1). a solution of acetic acid and sodium acetate (ch 3 cooh ch 3 coona) is an example of a buffer that consists. Substitute values into either form of the henderson hasselbalch approximation (equations 3.2.9 or 3.2.10) to calculate the ph. solution: according to the henderson hasselbalch approximation (equation 3.2.9), the ph of a solution that contains both a weak acid and its conjugate base is. ph = pka log([a −] [ha]). The first regime is a straightforward equilibrium problem. the second regime involves reaction of the acid and base as a limiting reagent problem, followed by straightforward equilibration of the resulting solution. the third regime is handled differently depending on whether the acid (or base) initially present is a weak or strong acid (or base). Definition. a buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. acidic buffer solutions. an acidic buffer solution is simply one which has a ph less than 7. acidic buffer solutions are commonly made from a weak acid and one of its salts often a sodium salt.
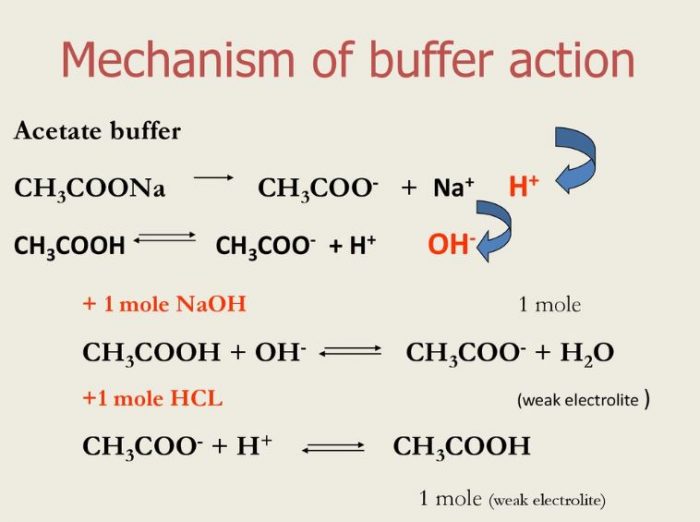
Buffer Solution And Buffer Action Chemistry Class 11 Ionic Equilibrium The first regime is a straightforward equilibrium problem. the second regime involves reaction of the acid and base as a limiting reagent problem, followed by straightforward equilibration of the resulting solution. the third regime is handled differently depending on whether the acid (or base) initially present is a weak or strong acid (or base). Definition. a buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. acidic buffer solutions. an acidic buffer solution is simply one which has a ph less than 7. acidic buffer solutions are commonly made from a weak acid and one of its salts often a sodium salt. Lecture 22: acid base equilibrium: salt solutions and buffers description: embedded video, no tabs, this description appears on section page: a buffer helps to maintain a constant ph. our blood has a natural buffering system to ensure that the ph of our blood stays within a narrow window and that we stay health. If you're seeing this message, it means we're having trouble loading external resources on our website. if you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.

Comments are closed.