Acids And Bases Buffer Calculation Past Paper Exam Question

Acids And Bases Buffer Calculation Past Paper Exam Question Ace your a level chemistry exams with this in depth analysis of a past paper question. i break down the complexities and guide you through every step to ensu. Print. all questions full mark scheme. question 1. mark scheme. next. past paper questions for the buffer action topic of a level aqa chemistry.

Acids And Bases Buffer Calculation Past Paper Questionпѕњa Tldr the video script is a detailed walkthrough of a buffer calculation question from an aqa a level chemistry past paper. it emphasizes the importance of starting with moles and understanding the equilibrium shifts when adding a strong base or acid to the solution. Answer: mixture of naclo 4 and hclo 4 is not an example of an acidic buffer solution. q4. a buffer solution is a mixture of. (a) weak acid and strong base. (b) strong acid and weak base. (c ) strong acid and its conjugate base. (d) weak acid and its conjugate base. answer: (d) buffer solution is a mixture of a weak acid and its conjugate base. 4.3 test (mark scheme) more exam questions on 4.3 acids and bases (mark scheme) 4.3 exercise 1 bronsted lowry theory. 4.3 exercise 2 ph calculations. 4.3 exercise 3 buffer solutions. 4.3 exercise 4 titrations and indicators. answers to 4.3 exercises. click here to view some great books which can aid your learning. note: all resources on. One mark for ph = 0.92 or ph = 5.15. (using incorrect ka values) examiner’s comments. most candidates could calculate the ph of a weak acid although a significant number gave the answer as 3.0, presumably confusing the demand for two decimal points with two significant figures. n(c2h5cooh) = (0.0800 ×. b i. and.
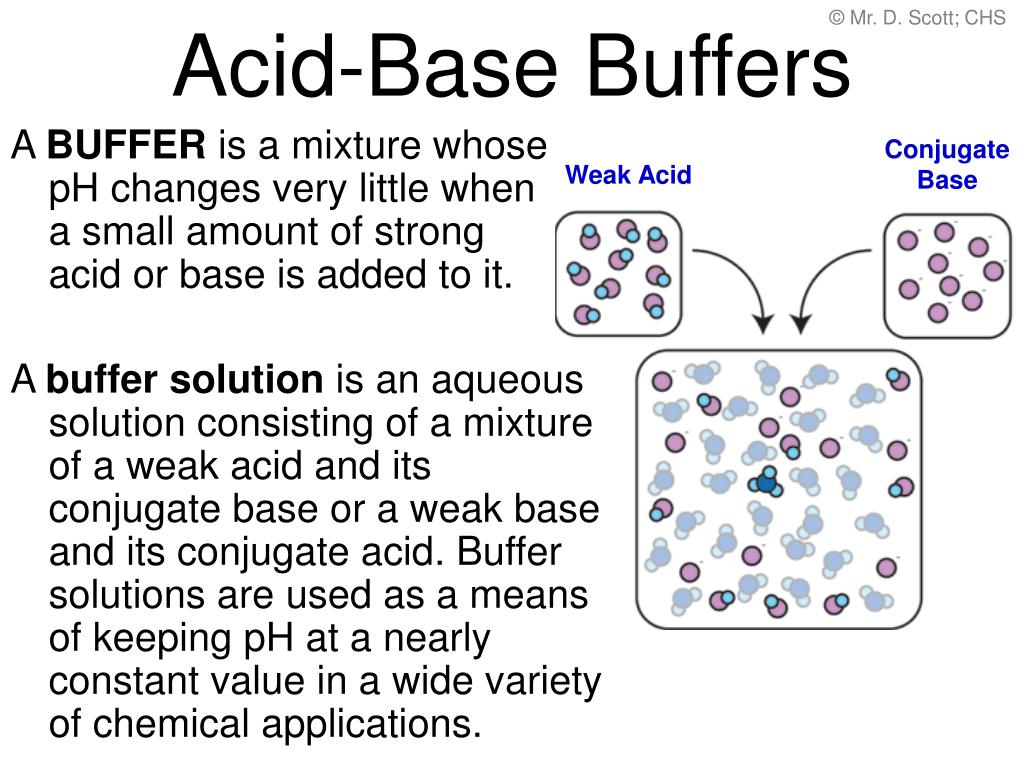
Ppt Acid Base Buffers Powerpoint Presentation Free Download Id 496487 4.3 test (mark scheme) more exam questions on 4.3 acids and bases (mark scheme) 4.3 exercise 1 bronsted lowry theory. 4.3 exercise 2 ph calculations. 4.3 exercise 3 buffer solutions. 4.3 exercise 4 titrations and indicators. answers to 4.3 exercises. click here to view some great books which can aid your learning. note: all resources on. One mark for ph = 0.92 or ph = 5.15. (using incorrect ka values) examiner’s comments. most candidates could calculate the ph of a weak acid although a significant number gave the answer as 3.0, presumably confusing the demand for two decimal points with two significant figures. n(c2h5cooh) = (0.0800 ×. b i. and. 1. 20 20 a student investigates the reactions of two weak monobasic acids: 2 hydroxypropanoic acid, ch3ch(oh)cooh, and butanoic acid, ch3ch2ch2cooh. (a) the student wants to prepare a standard solution of 2 hydroxypropanoic acid that has a ph of 2.19. plan how the student could prepare 250 cm3 of this standard solution from solid 2. Spanish language & literature. past papers. other subjects. revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save my exams.
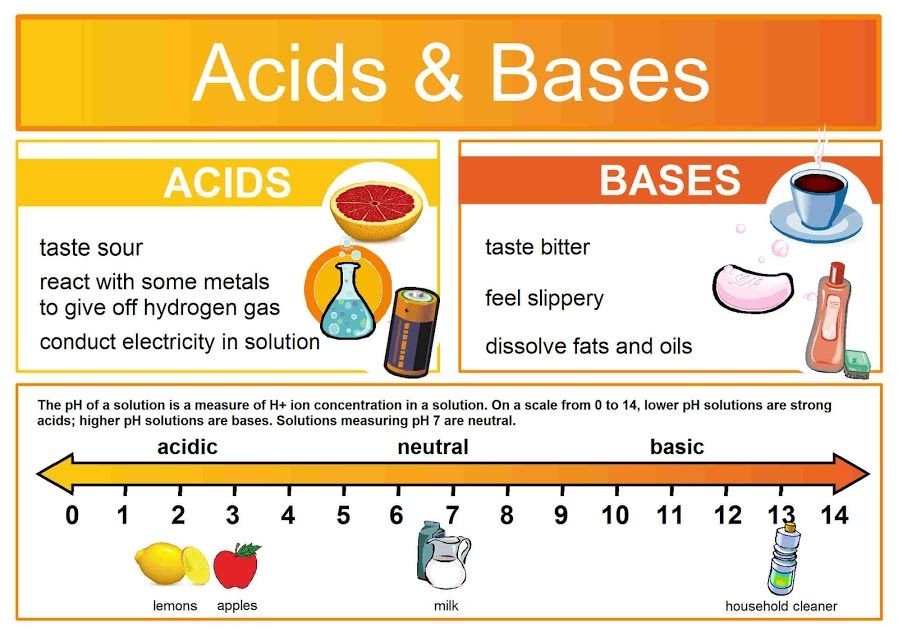
Scb 115 Lab 2 Exercise 4 Ph Acids Bases And Buffers Natural 1. 20 20 a student investigates the reactions of two weak monobasic acids: 2 hydroxypropanoic acid, ch3ch(oh)cooh, and butanoic acid, ch3ch2ch2cooh. (a) the student wants to prepare a standard solution of 2 hydroxypropanoic acid that has a ph of 2.19. plan how the student could prepare 250 cm3 of this standard solution from solid 2. Spanish language & literature. past papers. other subjects. revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save my exams.

Comments are closed.