Are Clinical Trials Safe

Are Clinical Trials Safe Florida Institute For Clinical Research Yes, some clinical trials use placebos. a placebo is a pill or other intervention that has no active ingredient or benefit but appears to be a genuine treatment. people in the trials don’t know. Clinical research involves studying health and illness in people through observational studies or clinical trials. participating in a trial or study has many potential benefits and also some possible risks. learn about the benefits and risks of participating in clinical research and how your safety is protected.
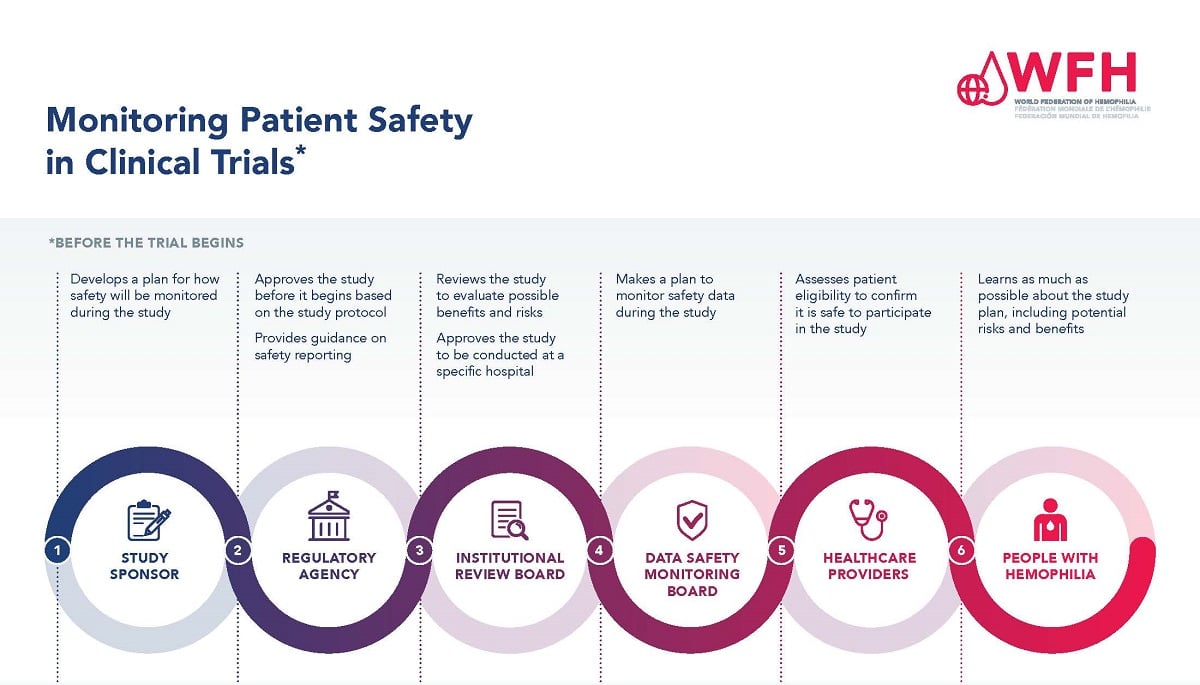
Monitoring Patient Safety In Clinical Trials Elearning Platform Clinical trials offer hope for many people while giving researchers a chance to find treatments that could benefit patients in the future. healthy volunteers say they take part to help others and contribute to moving science forward. people with an illness or disease may take part to help others but also to have a chance to receive the newest. Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current. Learn how clinical trials are designed and monitored to protect your safety and rights. find out about the roles of irbs, dsmbs, ohrp, fda, and more in ensuring the ethical and scientific quality of cancer research. Clinical trials are research studies that test a medical, surgical, or behavioral intervention in people. these trials are the primary way that researchers determine if a new form of treatment or prevention, such as a new drug, diet, or medical device (for example, a pacemaker), is safe and effective in people.

Comments are closed.