Atom 3d Model Orbital Spin

Atom 3d Model Orbital Spin Youtube The electron orbital simulator (eos) is a unity program that serves as a visual aid for learning the structure of the atom. on the left is a 3d model of the atom selected in the periodic table. using the dropdown, you may view a simplified model of it's electron cloud or a 3d representation of the bohr model. on the right is the electron. Simple atomic 3d animation with electrons in s orbitals 1 4 & view with s p d f orbitals included spin paths.

6 6 3d Representation Of Orbitals Chemistry Libretexts build an atom phet interactive simulations. There are a total of five d orbitals and each orbital can hold two electrons. the transition metal series is defined by the progressive filling of the 3d orbitals.these five orbitals have the following ml values: ml=0, ±1, ±2, s orbitals | 2p orbitals | 3p orbitals | 3d orbitals | 4f orbitals. comparison of 1s, 2s and 2p orbitals. Modified by joshua halpern (howard university) 6.6: 3d representation of orbitals is shared under a license and was authored, remixed, and or curated by libretexts. orbitals with l = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. Atomic orbitals are basic building blocks of atomic orbital model (or electron cloud or wave mechanics model), a modern framework for visualizing submicroscopic behavior of electrons in matter. in this model electron cloud of an atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler.

Orbital Model Of Atom 3d Model Cgtrader Modified by joshua halpern (howard university) 6.6: 3d representation of orbitals is shared under a license and was authored, remixed, and or curated by libretexts. orbitals with l = 0 are s orbitals and are spherically symmetrical, with the greatest probability of finding the electron occurring at the nucleus. Atomic orbitals are basic building blocks of atomic orbital model (or electron cloud or wave mechanics model), a modern framework for visualizing submicroscopic behavior of electrons in matter. in this model electron cloud of an atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler. The energy levels are labeled with an n value, where n = 1, 2, 3, …. generally speaking, the energy of an electron in an atom is greater for greater values of n. this number, n, is referred to as the principal quantum number. figure 5.7.1 5.7. 1: different energy levels are numbered by principal quantum numbers n. Figure \ (\pageindex {1}\): electron configuration of a ground state hydrogen atom depicted on an energy level diagram. the electron is represented by an arrow in the 1s orbital. on the energy level diagram in figure \ (\pageindex {1}\), the horizontal lines labeled 1s, 2s, 2p, etc. denote the spatial parts of the orbitals, and an arrow.
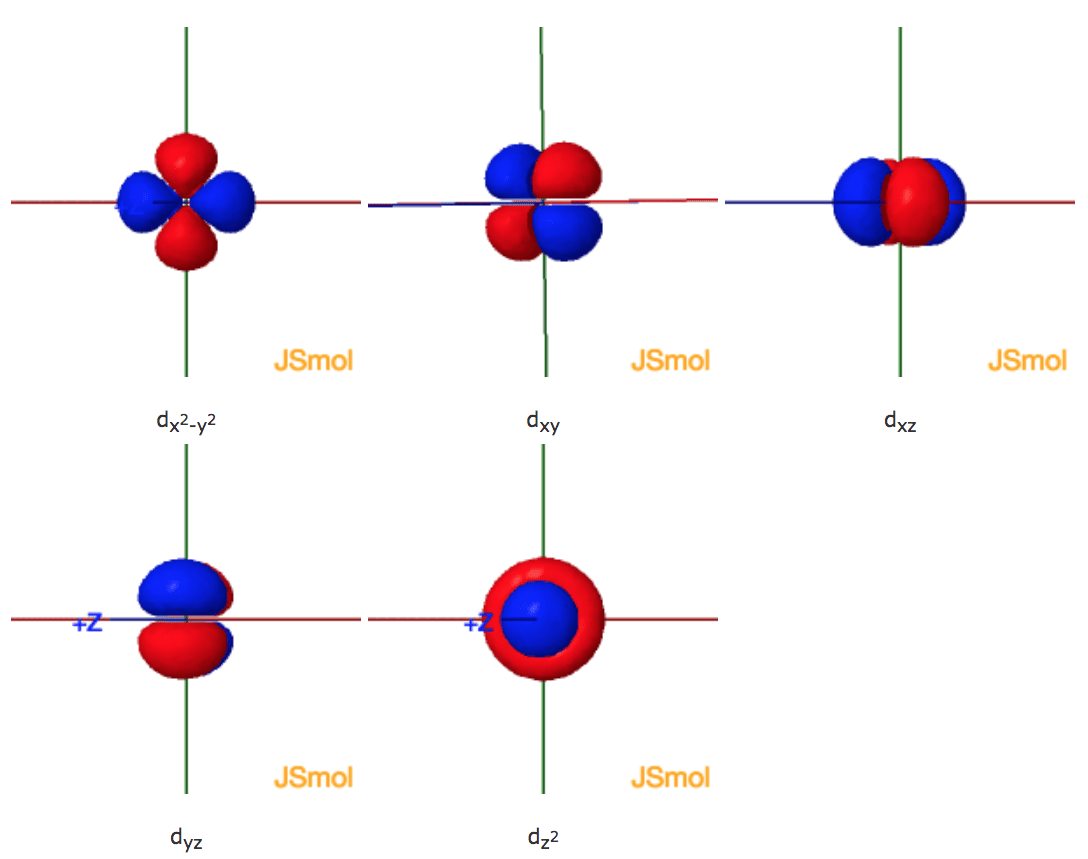
Shapes Of The 3d Orbitals In 3d The energy levels are labeled with an n value, where n = 1, 2, 3, …. generally speaking, the energy of an electron in an atom is greater for greater values of n. this number, n, is referred to as the principal quantum number. figure 5.7.1 5.7. 1: different energy levels are numbered by principal quantum numbers n. Figure \ (\pageindex {1}\): electron configuration of a ground state hydrogen atom depicted on an energy level diagram. the electron is represented by an arrow in the 1s orbital. on the energy level diagram in figure \ (\pageindex {1}\), the horizontal lines labeled 1s, 2s, 2p, etc. denote the spatial parts of the orbitals, and an arrow.

Comments are closed.