Avidity Drug For Muscular Dystrophy Shows Promise Syntis Takes New

юааavidityюабтащs Experimental Therapy юааshowsюаб юааpromiseюаб For Form Of юааmuscularюаб юааdystr Today, a brief rundown of news from avidity biosciences and nodthera, as well as updates from syntis bio, quralis and kyowa kirin that you may have missed from earlier this week. shares in avidity biosciences rose by nearly one third wednesday as the company revealed new clinical trial data for its drug del brax in a form of muscular dystrophy. New gene therapy approach shows promise for duchenne muscular dystrophy. sciencedaily . retrieved september 16, 2024 from sciencedaily releases 2024 07 240724191230.htm.
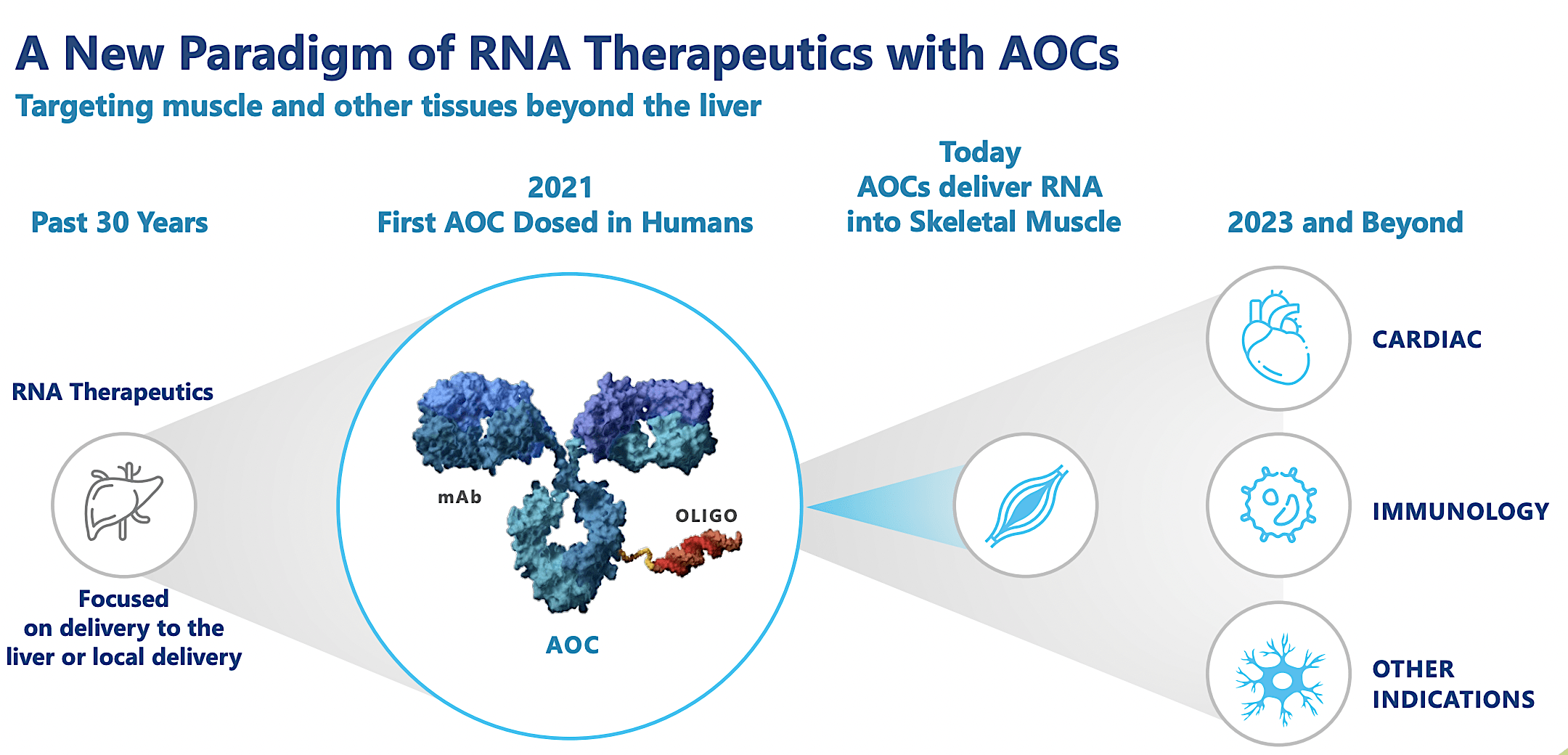
Avidity An Interesting Diversification Nasdaq Rna Seeking Alpha Early data for avidity's rna therapy show possible functional benefit in muscular dystrophy. avidity biosciences’ aoc 1020, newly dubbed del brax, demonstrated a safe profile and was tied to. Summary: a new gene therapy treatment for duchenne muscular dystrophy (dmd) shows promise of not only arresting the decline of the muscles of those affected by this inherited genetic disease, but. San diego based avidity aims to treat fshd by silencing the culprit gene. its therapy, formerly known as aoc 1020 and now renamed delpacibart braxlosiran (del brax), is part of a new class of rna. Avidity said its drug, called del brax, cut levels of that gene, called dux4, by an average of 53% across eight patients. it is the first trial to show significant reductions in gene expressions.

Comments are closed.