Binary Phase Diagrams
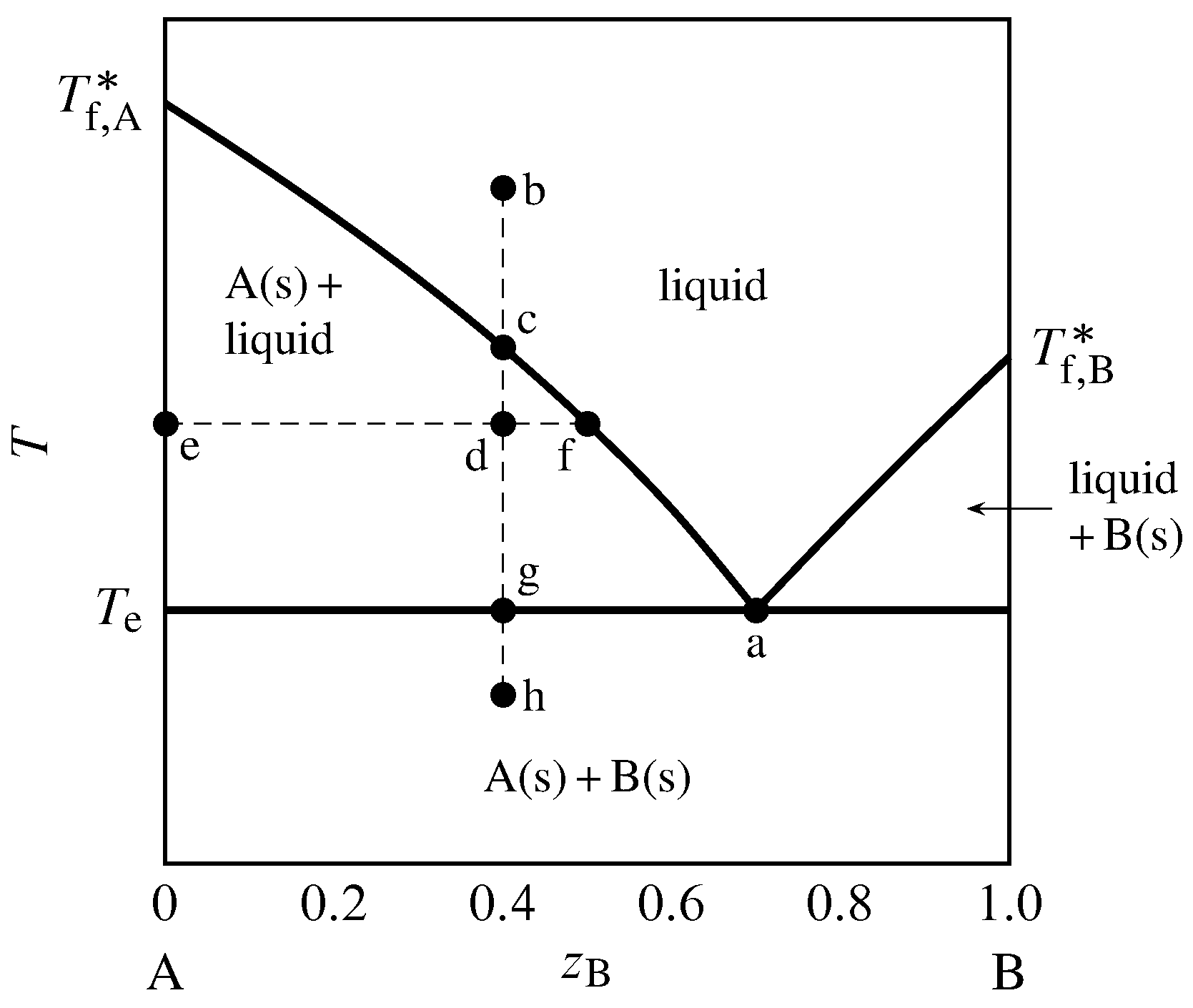
13 2 Phase Diagrams Binary Systems Chemistry Libretexts 13.2.2 solid–liquid systems. figure 13.1 temperature–composition phase diagram for a binary system exhibiting a eutectic point. figure 13.1 is a temperature–composition phase diagram at a fixed pressure. the composition variable is the mole fraction of component b in the system as a whole. 139. bi mass fraction of sn sn. 3 dimensional depiction of temperature composition phase diagram of bismuth, tin, and lead at 1atm. the diagram has been simplified by omission of the regions of solid solubility. each face of the triangular prism is a two component temperature composition phase diagram with a eutectic.
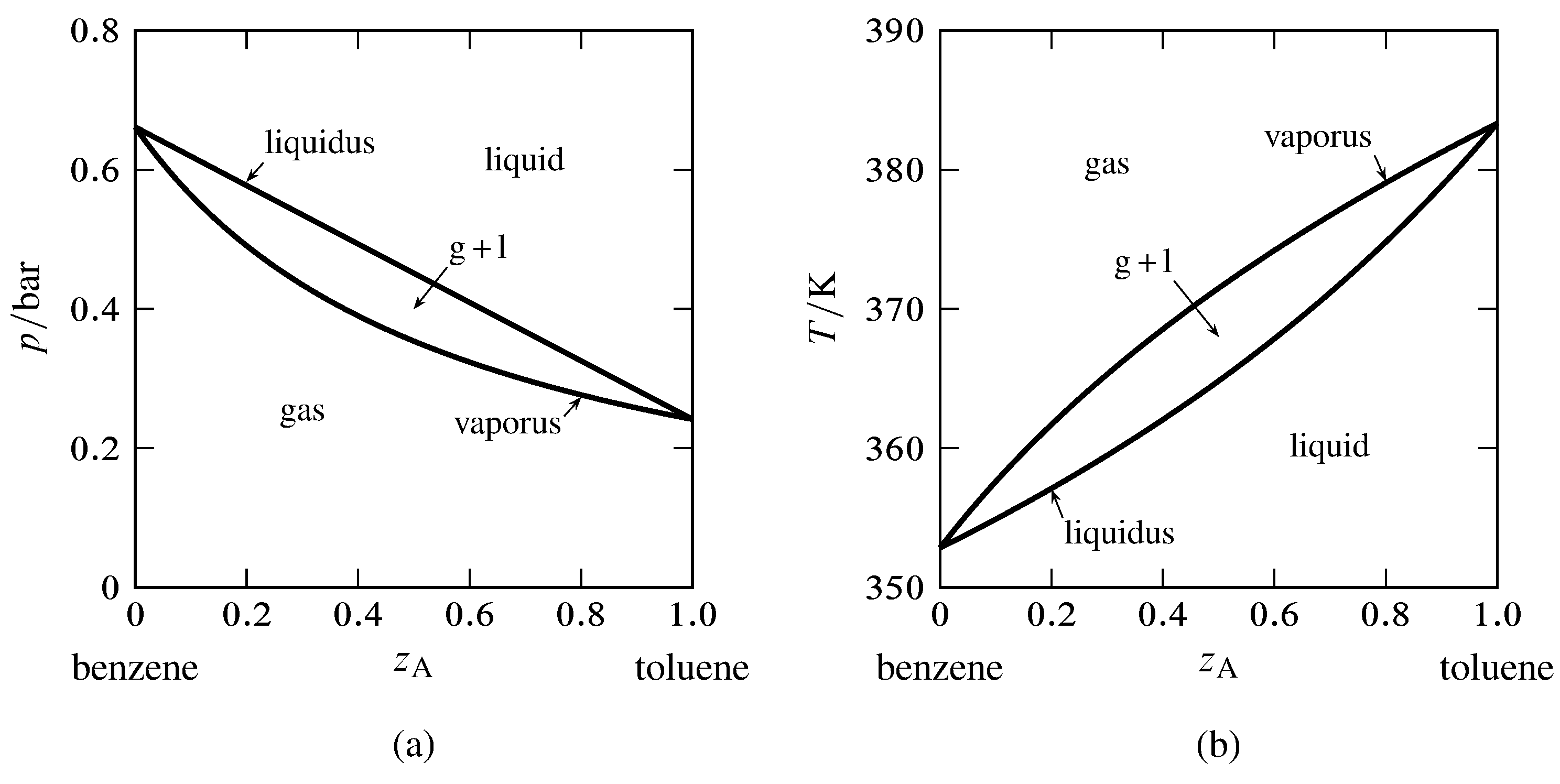
Phase Diagrams Binary Phase Diagrams Engineeringstuff A typical binary phase diagram (figure 1) indicates those phases present in equilibrium at any particular temperature and composition at the constant pressure for which the phase diagram was determined. at low temperatures, the only phase present is the solid designated by \(s\) in the phase diagram. Figure chapter4.7: eutectic phase diagram. the binary eutectic phase diagram has several distinctive features one being a solid solid phase mixture, limit of solubility at different temperatures, and an invariant point in the phase diagram, the eutectic point. the solubility limit is the maximum amount of solute that you can integrate into the. A phase diagram for a binary system displaying a eutectic point. other much more complex types of phase diagrams can be constructed, particularly when more than one pure component is present. in that case, concentration becomes an important variable. phase diagrams with more than two dimensions can be constructed that show the effect of more. Mse 2090: introduction to materials science chapter 9, phase diagrams 14 interpretation of a binary phase diagrams for a given temperature and composition we can use phase diagram to determine: 1) the phases that are present 2) compositions of the phases 3) the relative fractions of the phases finding the composition in a two phase region: 1.
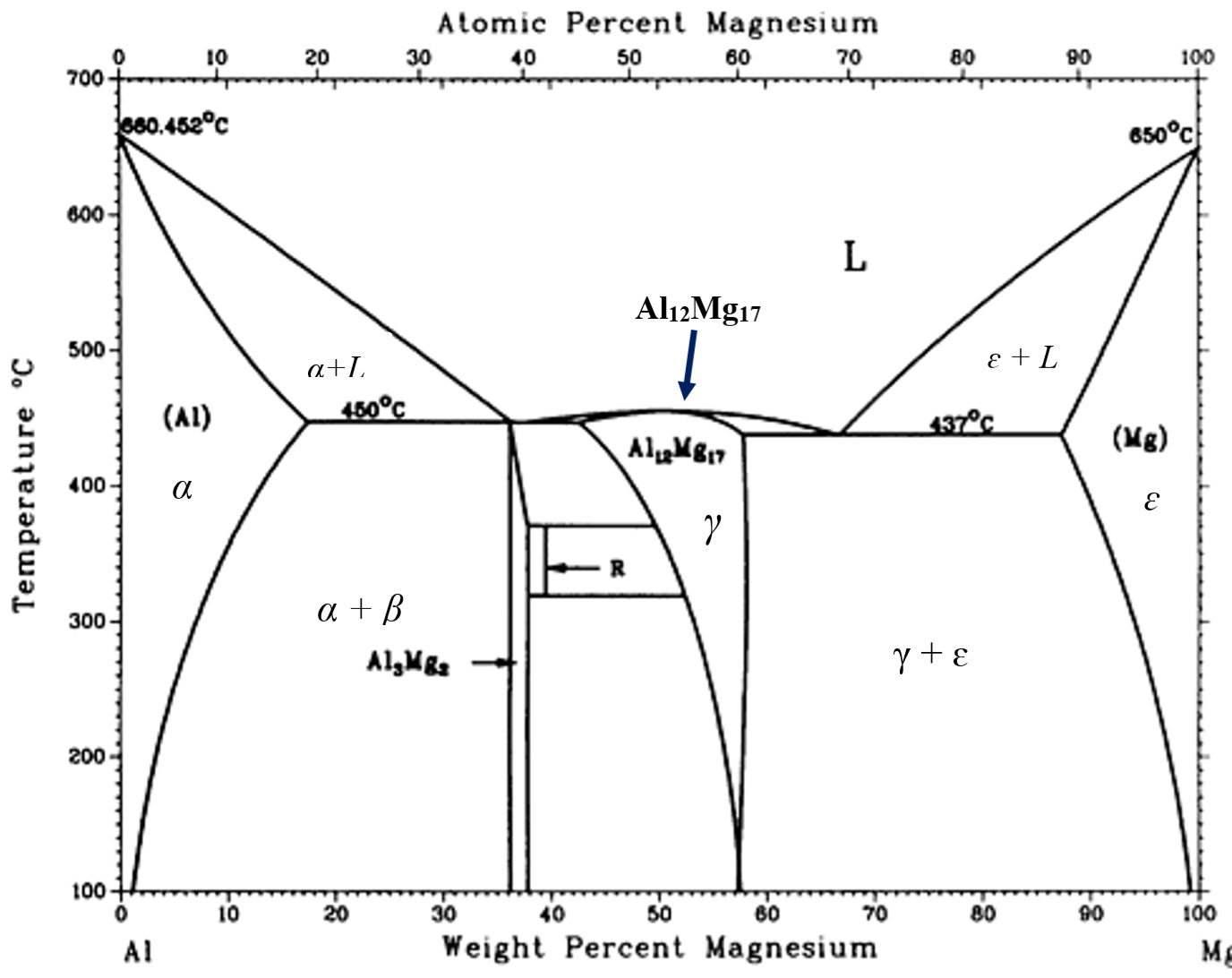
13 2 Phase Diagrams Binary Systems Chemistry Libretexts A phase diagram for a binary system displaying a eutectic point. other much more complex types of phase diagrams can be constructed, particularly when more than one pure component is present. in that case, concentration becomes an important variable. phase diagrams with more than two dimensions can be constructed that show the effect of more. Mse 2090: introduction to materials science chapter 9, phase diagrams 14 interpretation of a binary phase diagrams for a given temperature and composition we can use phase diagram to determine: 1) the phases that are present 2) compositions of the phases 3) the relative fractions of the phases finding the composition in a two phase region: 1. Learn how to read and interpret type 3 binary phase diagrams, which show partial solubility, change of state, and freezing point depression of components. see examples of type 3 diagrams for ethylene glycol water, chloride salts water, cubic zirconia, aluminum magnesium, lead tin, and iron sulfur solutions. Free energy diagrams define the structure of the phase diagram. compositions of two phase regions, which are tangent points on the free energy diagram, become phase boundaries on the phase diagram. the lever rule is directly applied to tie lines on the phase diagram, to determine the amount of each phase present in two phase regions.
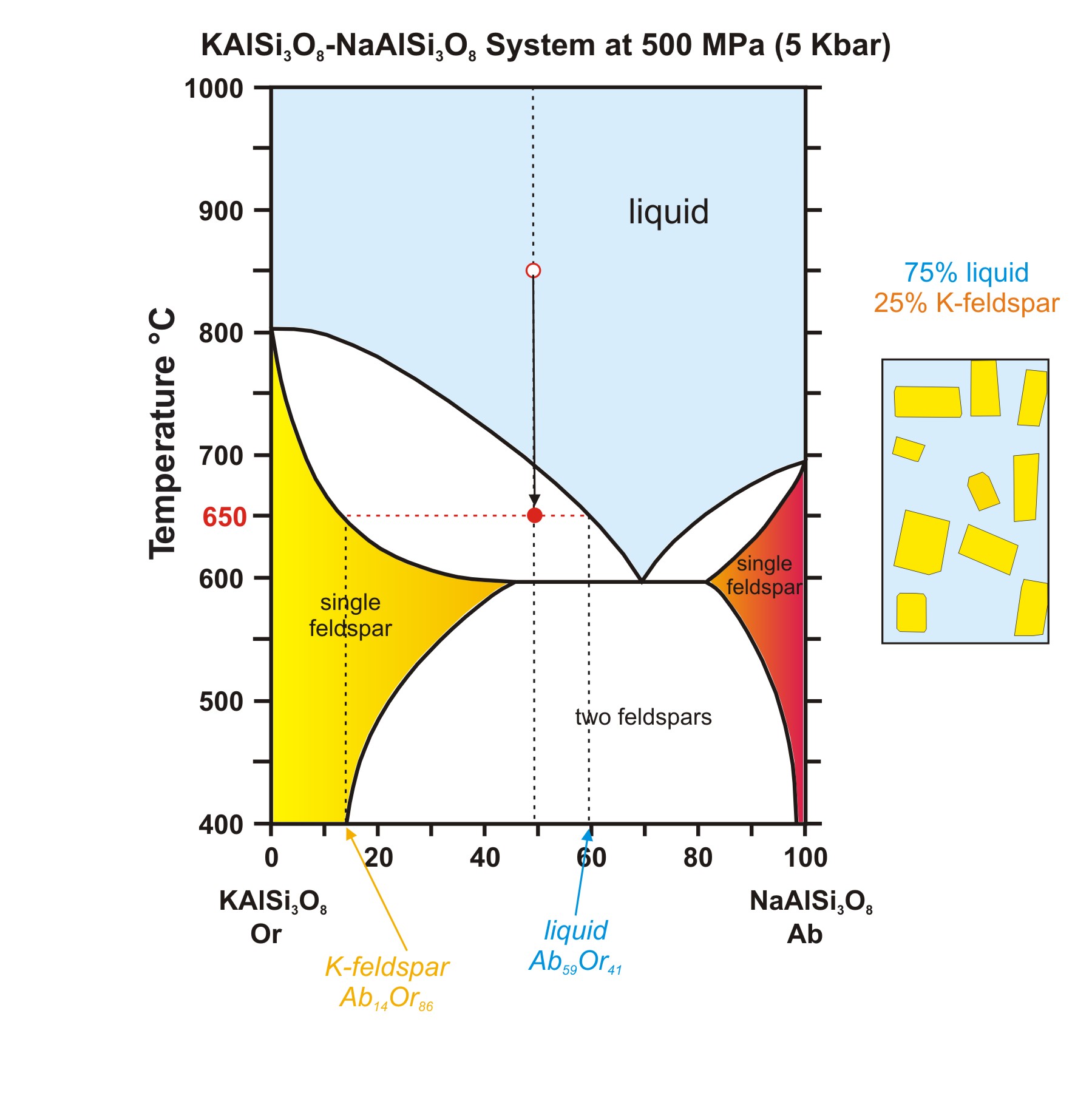
Binary Phase Diagrams Learn how to read and interpret type 3 binary phase diagrams, which show partial solubility, change of state, and freezing point depression of components. see examples of type 3 diagrams for ethylene glycol water, chloride salts water, cubic zirconia, aluminum magnesium, lead tin, and iron sulfur solutions. Free energy diagrams define the structure of the phase diagram. compositions of two phase regions, which are tangent points on the free energy diagram, become phase boundaries on the phase diagram. the lever rule is directly applied to tie lines on the phase diagram, to determine the amount of each phase present in two phase regions.

Binary Phase Diagrams Explained Youtube

Comments are closed.