Calculation Of Percentage Ionization Of Weak Electrolytes

Calculation Of Percent Ionized Of Weak Electrolytes Youtube Figure 16.6.5: ph paper indicates that a 0.1 m solution of nh 3 (left) is weakly basic. the solution has a poh of 3 ( [oh −] = 0.001 m) because the weak base nh 3 only partially reacts with water. a 0.1 m solution of naoh (right) has a poh of 1 because naoh is a strong base (credit: modification of work by sahar atwa). Now, percent ionization or percent association represents the percentage of we of a weak acid that can become ionized when placed in an aqueous solution. remember that weak acids represent weak electrolytes and strong acids represent strong electrolytes. because of this weak acids ionize less than 100% when placed in an aqueous solution.
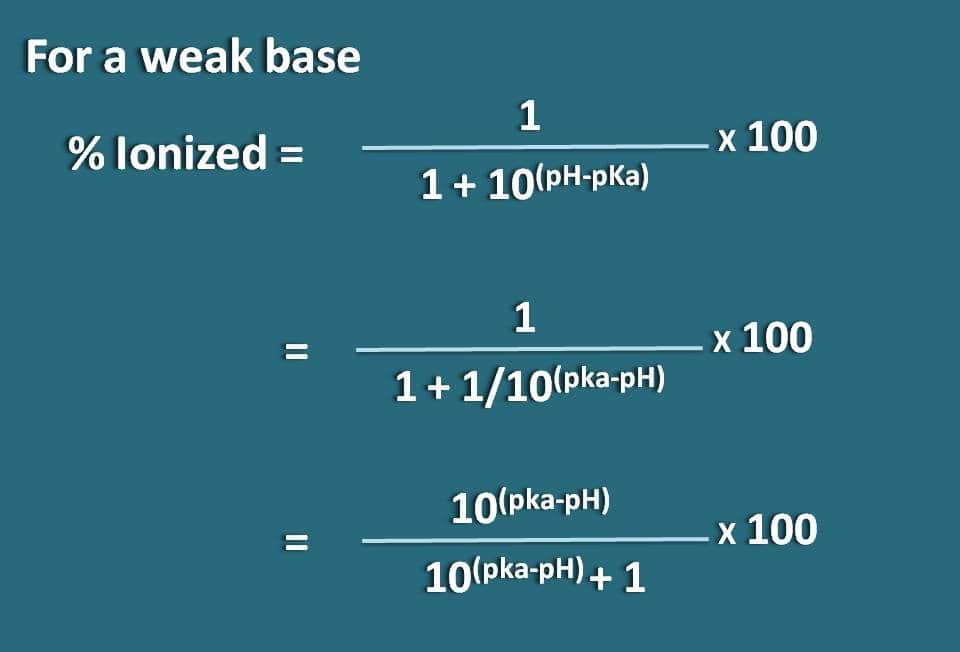
Calculation Of Percentage Ionization Of Weak Electrolytes Hso − 4(aq) h2o(aq) ⇌ h3o (aq) so2 − 4 (aq)ka = 1.2 × 10 − 2. another measure of the strength of an acid is its percent ionization. the percent ionization of a weak acid is the ratio of the concentration of the ionized acid to the initial acid concentration, times 100: % ionization = [h3o ]eq [ha] 0 × 100. Step 1: read through the given information to find the initial concentration and the equilibrium constant for the weak acid or base. c n h 3 = 0.12 and k b = 1.8 × 10 − 5. step 2: use the. Distinguish between strong and weak electrolytes. explain what happens when electrolytes dissolve in water. give the equilibrium constant expression for ionizaton. explain ion product of water, autoionization of water, and ph. calculate ionization percentage of weak electrolytes. explain metathesis reactions. Now let's let ha ionize by 10% which is actually on the strong end of weak acids. do the math. if 10% ionizes, then you make 0.010 m h and 0.010 m a –, while leaving behind (unionized) 90% which is 0.090 m ha. you now have all three concentrations to plug into the ka expression and calculate its value: k a = 0.01 2 0.090 = 1.1 × 10 − 3.
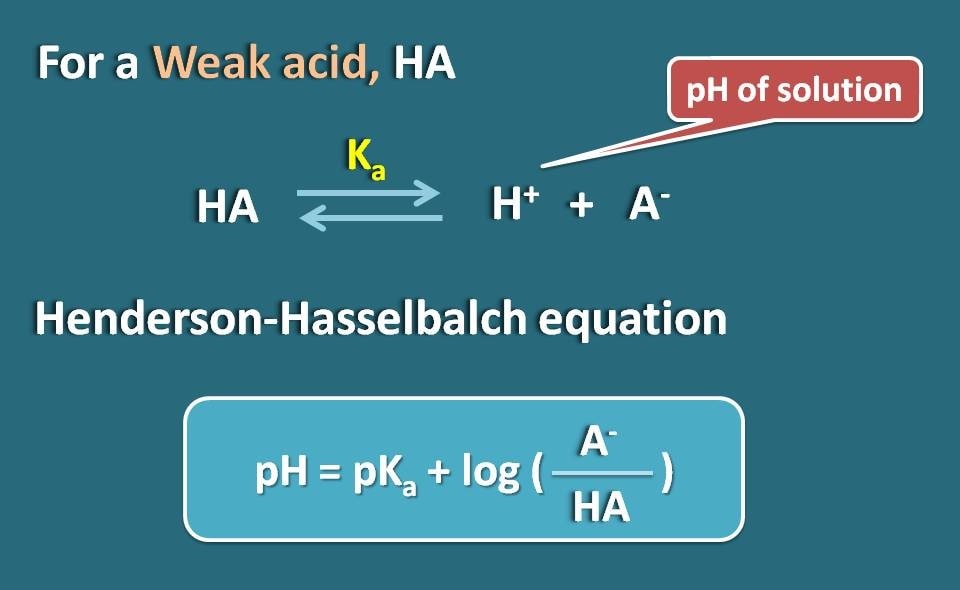
Calculation Of Percentage Ionization Of Weak Electrolytes Distinguish between strong and weak electrolytes. explain what happens when electrolytes dissolve in water. give the equilibrium constant expression for ionizaton. explain ion product of water, autoionization of water, and ph. calculate ionization percentage of weak electrolytes. explain metathesis reactions. Now let's let ha ionize by 10% which is actually on the strong end of weak acids. do the math. if 10% ionizes, then you make 0.010 m h and 0.010 m a –, while leaving behind (unionized) 90% which is 0.090 m ha. you now have all three concentrations to plug into the ka expression and calculate its value: k a = 0.01 2 0.090 = 1.1 × 10 − 3. The curvature of the plots for intermediate electrolytes is a simple consequence of the le chatelier effect, which predicts that the equilibrium. mx(aq) = m (aq) x–(aq) (8.10.9c.1) (8.10.9c.1) m x (a q) = m (a q) x (a q) –. will shift to the left as the concentration of the "free" ions increases. in more dilute solutions, the actual. This raises the level of hydronium ion in an aqueous solution of weak acid. example 1. calculating percent ionization of a weak acid. if acetic acid (ch3cooh) has a ka of 1.8*10 5 at 250c, what is the percent ionization of acetic acid in a 1.00 m solution? let’s go through this example step by step.
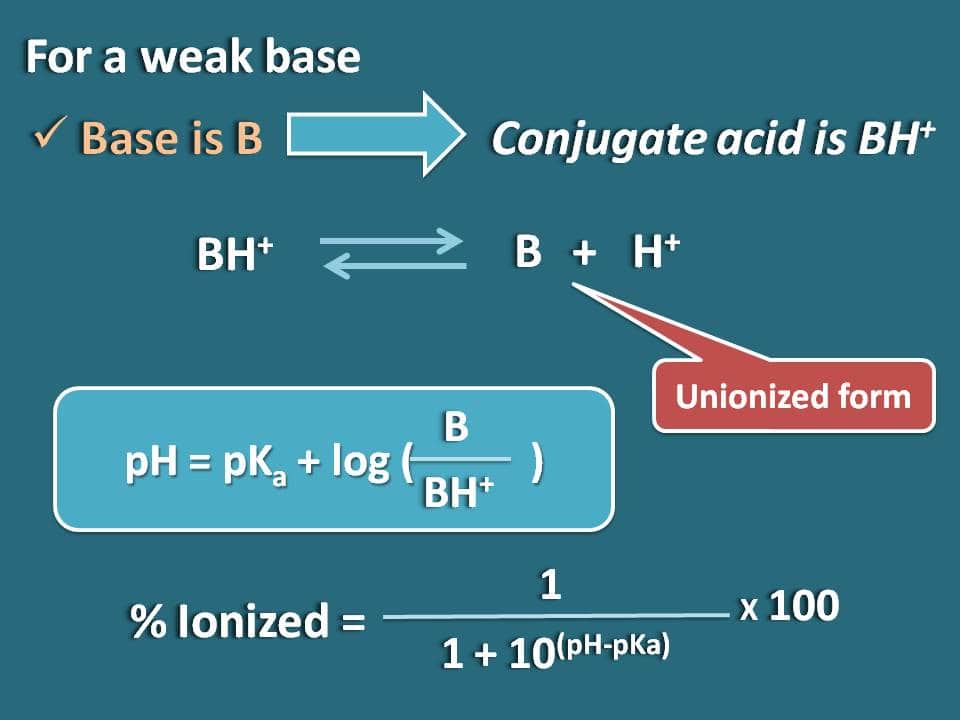
Calculation Of Percentage Ionization Of Weak Electrolytes The curvature of the plots for intermediate electrolytes is a simple consequence of the le chatelier effect, which predicts that the equilibrium. mx(aq) = m (aq) x–(aq) (8.10.9c.1) (8.10.9c.1) m x (a q) = m (a q) x (a q) –. will shift to the left as the concentration of the "free" ions increases. in more dilute solutions, the actual. This raises the level of hydronium ion in an aqueous solution of weak acid. example 1. calculating percent ionization of a weak acid. if acetic acid (ch3cooh) has a ka of 1.8*10 5 at 250c, what is the percent ionization of acetic acid in a 1.00 m solution? let’s go through this example step by step.

Comments are closed.