Chemical Classification Of Matter Part 3 Some Basic Concepts Of

Chemical Classification Of Matter Part 3 Some Basic Concepts Of Modified by joshua halpern (howard university) is shared under a license and was authored, remixed, and or curated by libretexts. matter can be classified according to physical and chemical properties. matter is anything that occupies space and has mass. the three states of matter are solid, liquid, and gas. a physical change …. Phases. another way to classify matter is to describe it as a solid, a liquid, or a gas, which was done in the examples of solutions. these three descriptions, each implying that the matter has certain physical properties, represent the three phases of matter. a solid has a definite shape and a definite volume.
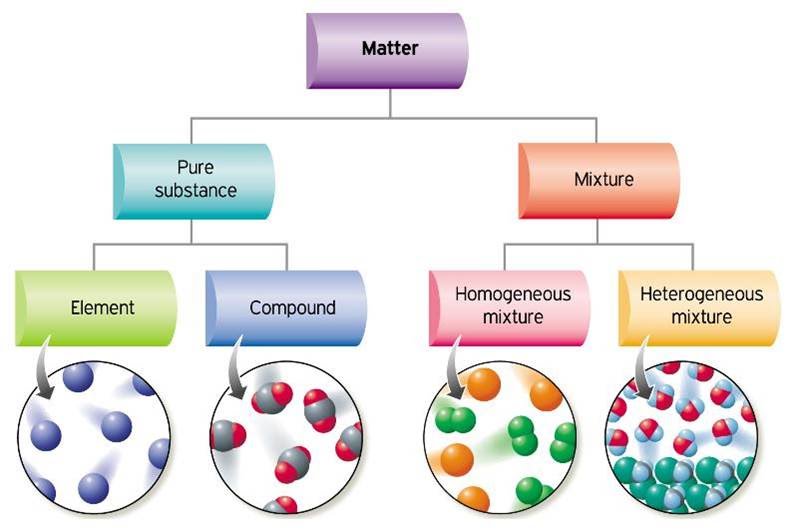
Classification Of Matter Chemistry 10 Chemical properties are characteristics that describe how matter changes its chemical structure or composition. an example of a chemical property is flammability—a material’s ability to burn—because burning (also known as combustion) changes the chemical composition of a material. oxidation, rusting, decomposition, and inertness are. Figure \(\pageindex{3}\) illustrates the relationships between the different ways matter can be classified. figure \(\pageindex{6}\): the classification of matter. matter can be classified in a variety of ways, depending on its properties. this table starts with a substance. if there is only one present, it can either be an element or a compound. A gas takes both the shape and volume of its container. figure 1.2.1 the three most common states or phases of matter are solid, liquid, and gas. a fourth state of matter, plasma, occurs naturally in the interiors of stars. a plasma is a gaseous state of matter that contains appreciable numbers of electrically charged particles (figure 1.2.2). A molecule is a combination of atoms. matter can be classified as either pure substances (elements and compounds) or mixtures (homogeneous and heterogeneous mixtures), depending on their characteristics. all substances have distinct physical and chemical properties, and may undergo physical or chemical changes.
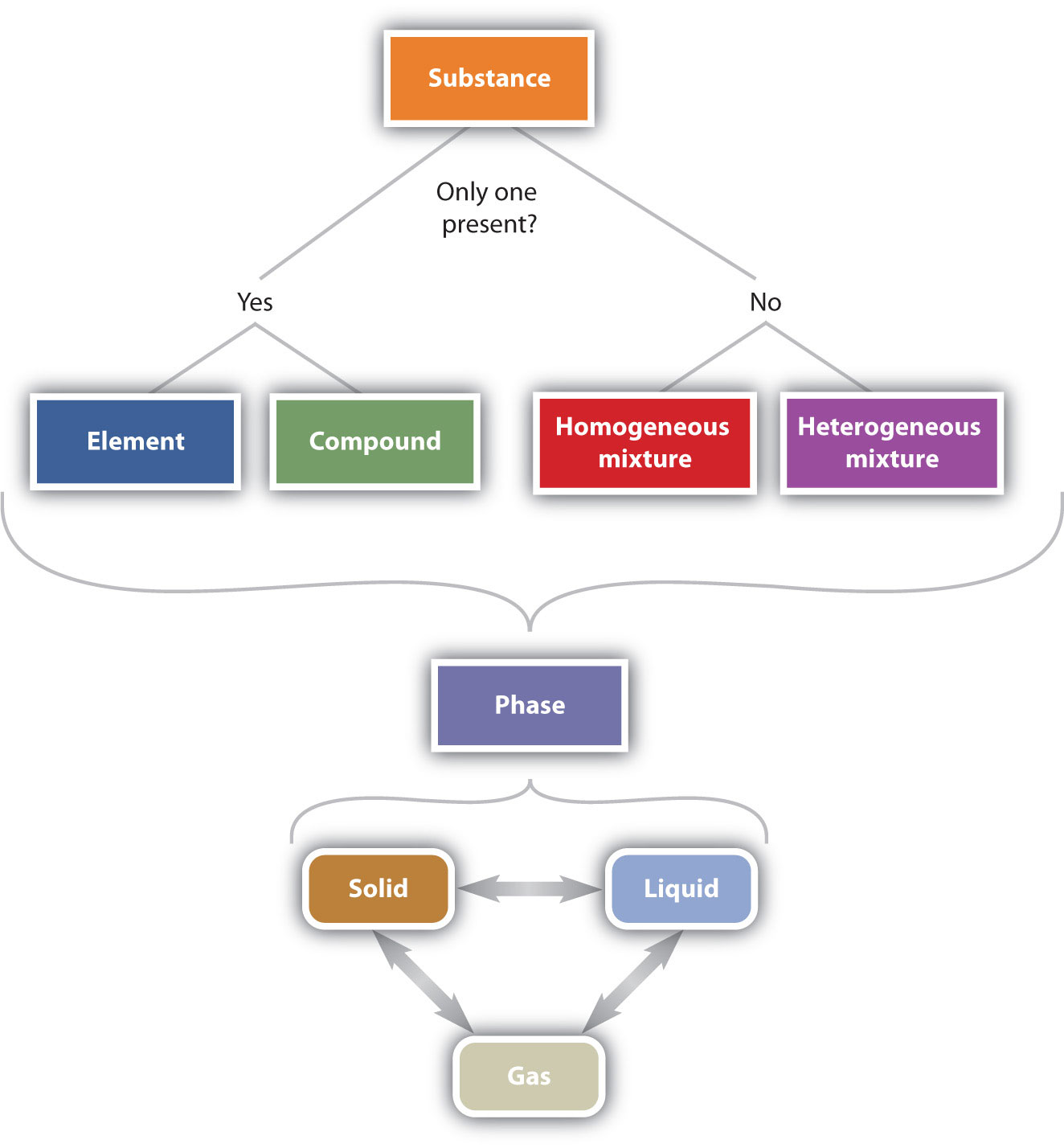
1 2 The Classification Of Matter Chemistry Libretexts A gas takes both the shape and volume of its container. figure 1.2.1 the three most common states or phases of matter are solid, liquid, and gas. a fourth state of matter, plasma, occurs naturally in the interiors of stars. a plasma is a gaseous state of matter that contains appreciable numbers of electrically charged particles (figure 1.2.2). A molecule is a combination of atoms. matter can be classified as either pure substances (elements and compounds) or mixtures (homogeneous and heterogeneous mixtures), depending on their characteristics. all substances have distinct physical and chemical properties, and may undergo physical or chemical changes. A fourth state of matter, plasma, occurs naturally in the interiors of stars. a plasma is a gaseous state of matter that contains appreciable numbers of electrically charged particles . the presence of these charged particles imparts unique properties to plasmas that justify their classification as a state of matter distinct from gases. The classification of matter refers to the categorization of substances based on their physical and chemical properties. matter can be classified into pure substances and mixtures. pure substances have a fixed composition and can be elements or compounds. elements are the simplest form of matter that can't be broken down by chemical means and.

Basic Chemistry Concepts Matter Element Atom Molecule Compound A fourth state of matter, plasma, occurs naturally in the interiors of stars. a plasma is a gaseous state of matter that contains appreciable numbers of electrically charged particles . the presence of these charged particles imparts unique properties to plasmas that justify their classification as a state of matter distinct from gases. The classification of matter refers to the categorization of substances based on their physical and chemical properties. matter can be classified into pure substances and mixtures. pure substances have a fixed composition and can be elements or compounds. elements are the simplest form of matter that can't be broken down by chemical means and.

Classification Of Matter Chemistry Dictionary

Comments are closed.