Chemistry Conversion Chart Cheat Sheet
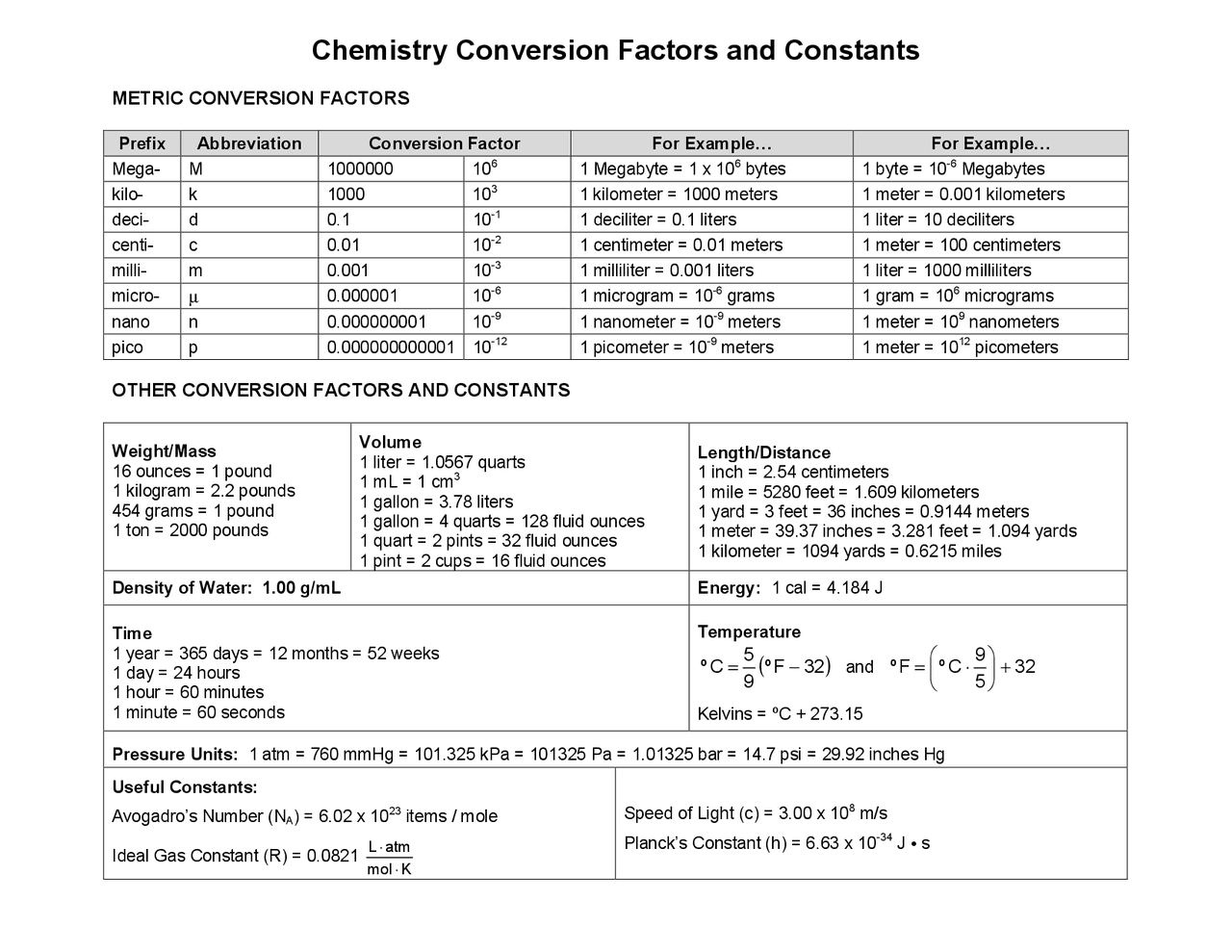
Cheat Sheet Chemistry Conversion Factors And Constants Cheat Sheetо Basic conversion cheat sheet. three basic units of measurement length, mass (weight), volume. the basic unit of length is: meter. the basic unit of volume is: liter. the basic unit of mass (weight) is: gram. the following are some of the prefixes for the metric system. they are based on powers of ten and can all be applied to length, mass, or. Following are some important conversions of temperature, size, and pressure as well as metric prefixes to memorize for your chemistry class: temperature conversions: °f = 9 5(°c) 32 °c = 5 9(°f – 32) k = °c 273. english metric conversions: 1 in = 2.54 cm. 1 lb = 454 g. 1 qt = 0.946 l. pressure conversion: 1atm = 760 mmhg = 760 torr.

Cheat Sheet Chemistry Conversion Chart English units. lb = pound ounce vs fluid ounce: oz is a mass unit and fl oz is a volume unit in the english system. oz = ounce pt = pint qt = quart. Common chemistry conversions english to metric conversions (mass, length, volume, and area conversions are good to 4 significant figures) mass length volume area temperature 1 lb = 453.6 g 1 in = 2.540 cm 1 fl oz = 29.57 ml 1 in2 = (2.54 cm)2 =6.452 cm2 t°c = 5 9 (t°f 32). Suppose you wish to convert pressure of 14 lb in 2 to g cm 2. when setting up the conversion, worry about one unit at a time, for example, convert the pound units to gram units, first: next, convert in 2 to cm 2. set up the conversion without the exponent first, using the conversion factor, 1 in = 2.54 cm. A polynomial equation of the second degree in the form of ax2 bx c = 0. 2 equation: ax2 bx c=0 roots: x = − b ± b − 4 ac. 2 a. it always has two roots (or solutions) x1 & x2. for most chemical problems (mass, temperature, concentration etc.), ignore the negative root. example: equilibrum concentration equation x2 3x 10 = 0.
Chemistry Conversion Chart Cheat Sheet Suppose you wish to convert pressure of 14 lb in 2 to g cm 2. when setting up the conversion, worry about one unit at a time, for example, convert the pound units to gram units, first: next, convert in 2 to cm 2. set up the conversion without the exponent first, using the conversion factor, 1 in = 2.54 cm. A polynomial equation of the second degree in the form of ax2 bx c = 0. 2 equation: ax2 bx c=0 roots: x = − b ± b − 4 ac. 2 a. it always has two roots (or solutions) x1 & x2. for most chemical problems (mass, temperature, concentration etc.), ignore the negative root. example: equilibrum concentration equation x2 3x 10 = 0. Factors for unit conversions. prof. faith a. morrison department of chemical engineering. quantity. equivalent values. mass. 1 kg = 1000 g = 0.001 metric ton = 2.20462 lbm = 35.27392 oz. 1 lbm = 16 oz = 5 x 10‐4 ton = 453.593 g = 0.453593 kg. length. 10 12. 1 picometer (pm) = 10 12 meters. femto. f. 10 15. 1 femtometer (fm) = 10 15 meters. these base si units can be combined with any of the prefixes to create units that are most appropriate for what is being measured. for example, you wouldn’t measure the distance from la to new york in meters, the base unit.

Comments are closed.