Class 11 Chemistry Chapter 1 Some Basic Concepts Of Chemistry

Ncert Solutions For Class 11 Chemistry Chapter 1 Some Basicођ Free ncert solutions for class 11 chemistry chapter 1 some basic concepts of chemistry solved by expert teachers from latest edition books and as per ncert (cbse) guidelines.class 11 chemistry some basic concepts of chemistry ncert solutions and extra questions with solutions to help you to revise complete syllabus and score more marks. Ncert solutions class 11 chemistry chapter 1 – free pdf download. ncert solutions for class 11 chemistry chapter 1 some basic concepts of chemistry are provided on this page for class 11 chemistry students. these solutions can also be downloaded in a pdf format for free by clicking the download button provided below.

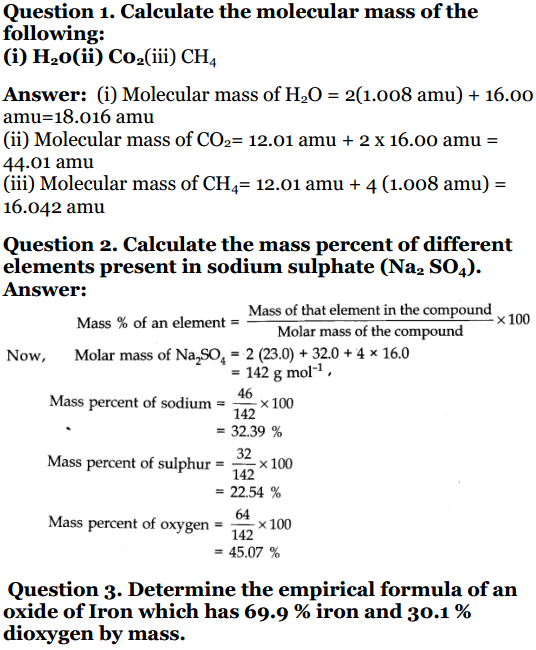
Class 11 Chemistry Worksheet On Chapter 1 Some Basic Co 4 chemistry 1.1 importance of chemistry chemistry plays a central role in science and is often intertwined with other branches of science. principles of chemistry are applicable in diverse areas, such as weather patterns, functioning of brain and operation of a computer, production in chemical industries,. The cbse chemistry chapter 1, some basic concepts of chemistry class 11 notes introduces fundamental concepts crucial for understanding the subject. it covers essentials like matter, its properties, and measurement units. focus on comprehending the mole concept, which plays a pivotal role in chemical calculations. Some basic concepts of chemistry class 11 notes chapter 1. chemistry has a direct impact on our life and has wide range of applications in different fields. these are given below: (ii) it has helped to protect the crops from insects and harmful bacteria, by the use ‘ of certain effective insecticides, fungicides and pesticides. Solution: molecular mass of na 2 so 4 = 2 × atomic mass of na atomic mass of s 4 × atomic mass of o. = 2 × 23 32 4 × 16 = 46 32 64 = 142 u. question 3. determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% oxygen by mass. solution: hence, the empirical formula is fe 2 co 3.

Ncert Book Class 11 Chemistry Chapter 1 Some Basic Conc Some basic concepts of chemistry class 11 notes chapter 1. chemistry has a direct impact on our life and has wide range of applications in different fields. these are given below: (ii) it has helped to protect the crops from insects and harmful bacteria, by the use ‘ of certain effective insecticides, fungicides and pesticides. Solution: molecular mass of na 2 so 4 = 2 × atomic mass of na atomic mass of s 4 × atomic mass of o. = 2 × 23 32 4 × 16 = 46 32 64 = 142 u. question 3. determine the empirical formula of an oxide of iron which has 69.9% iron and 30.1% oxygen by mass. solution: hence, the empirical formula is fe 2 co 3. With a focus on clarity and depth, these resources serve as indispensable tools for students navigating through chemistry class 11, chapter 1 of their chemistry syllabus. 1. class 11 chemistry chapter 1 ncert solutions free pdf download. 2. quick insights of “some basic concepts of chemistry” class 11 ncert solutions. Ans: the balanced equation for the combustion of carbon in dioxygen air is. (i) c o 2 = co 2. 1 mole of carbon burnt in air will produce 44 grams of co₂. (ii) 1 mole of carbon is burnt in 16g of dioxygen. 16g of dioxygen corresponds to 16 32 = 0.5 moles. i.e., dioxygen is the limiting reactant.

Class 11 Chemistry Revision Notes For Chapter 1 Some Basic With a focus on clarity and depth, these resources serve as indispensable tools for students navigating through chemistry class 11, chapter 1 of their chemistry syllabus. 1. class 11 chemistry chapter 1 ncert solutions free pdf download. 2. quick insights of “some basic concepts of chemistry” class 11 ncert solutions. Ans: the balanced equation for the combustion of carbon in dioxygen air is. (i) c o 2 = co 2. 1 mole of carbon burnt in air will produce 44 grams of co₂. (ii) 1 mole of carbon is burnt in 16g of dioxygen. 16g of dioxygen corresponds to 16 32 = 0.5 moles. i.e., dioxygen is the limiting reactant.
Ncert Solutions For Class 11 Chemistry Chapter 1 Some Basicођ

Comments are closed.