Clinical Trial Phases вђ James Lind Institute Public Health School In

Clinical Trial Phases вђ James Lind Institute Public He Phases of clinical research trials investigate a health intervention and collect sufficient evidence to declare it safe and efficacious. the process starts withdrug designanddiscovery followed by animal testing and then proceeds to human trials. the clinical trial initiates with safety tests followed by efficacy studies in human subjects. This has created a demand for skilled workforce in the clinical research industry as stressed in many business reports. overview james lind institute, through a variety of high quality clinical research training courses, is committed to developing emerging professionals for the clinical research industry who are equipped with the most relevant.

Adaptive Design Clinical Trials вђ James Lind Institute Public о James lind institute (jli), switzerland we are a swiss eduqua certified international educational institution based in geneva, switzerland. we prepare our students for careers in public health, health administration, pharmaceutical sciences, clinical research, economics, diplomacy, business management and more. Maximum allowed duration: 24 months. expected effort: 10 15 hours week. this advanced clinical trial management program is recommended for everyone already working or planning to work with clinical data and other related health databases. in this program, we aim to provide you with an exciting and pleasant experience so as to transform you into. M.sc. m.sc in clinical research. an initial deposit of €900.00 after receiving an offer of admission; followed by 11 ‘monthly installments’ of €390.00. this program requires a bachelor’s degree or an equivalent recognized academic title, preferably in a life science related area. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and summary results information for completed or terminated clinical studies. a study with results available on clinicaltrials.gov is described as having the results "posted.".
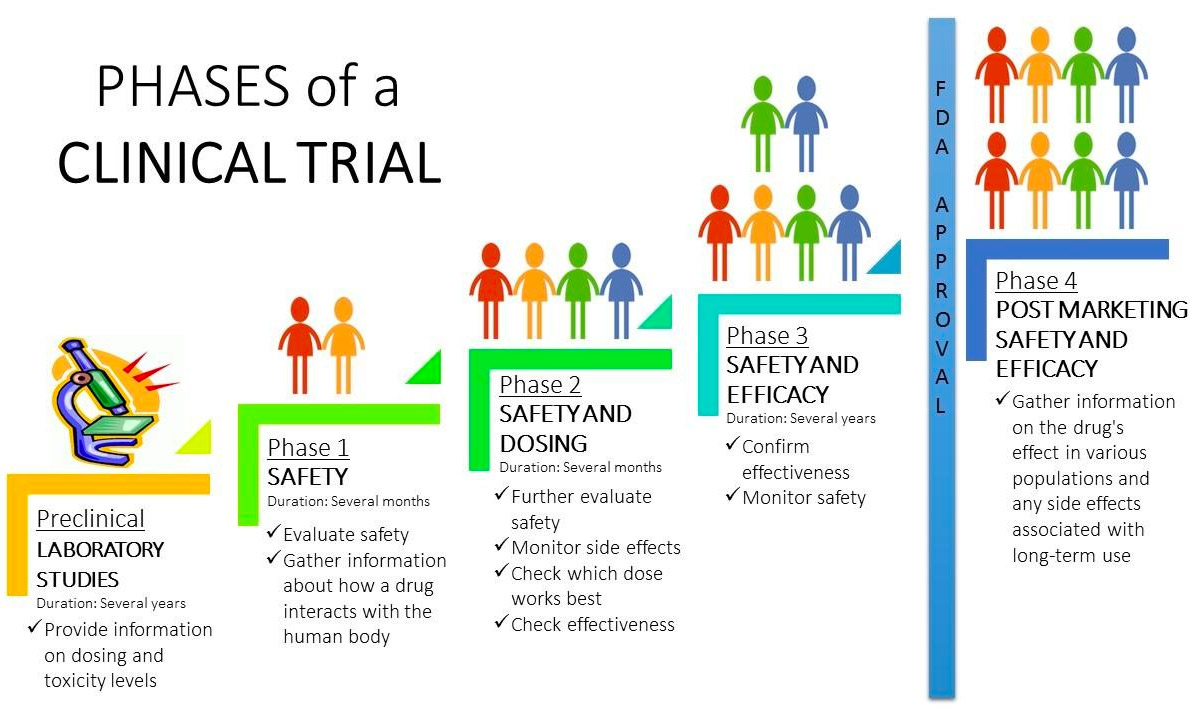
Clinical Trial Phases Diagram M.sc. m.sc in clinical research. an initial deposit of €900.00 after receiving an offer of admission; followed by 11 ‘monthly installments’ of €390.00. this program requires a bachelor’s degree or an equivalent recognized academic title, preferably in a life science related area. A structured online system, such as the clinicaltrials.gov results database, that provides the public with access to registration and summary results information for completed or terminated clinical studies. a study with results available on clinicaltrials.gov is described as having the results "posted.". The master of public health (mph), is a swiss degree awarded by james lind institute, switzerland. james lind institute is an approved post secondary higher educational institution with the authority to award private degrees in switzerland. the institute is registered in the canton of geneva, switzerland under the uid che 255.747.977. In may 1750 he was elected a fellow of the royal college of physicians of edinburgh (rcpe minutes 1.iv 1750). subsequent minutes of the college show that he attended meetings regularly and the 1756 minutes record lind’s appointment as college treasurer (rcpe minutes 2.xii.1756). he was also the college’s representative on the board of the.
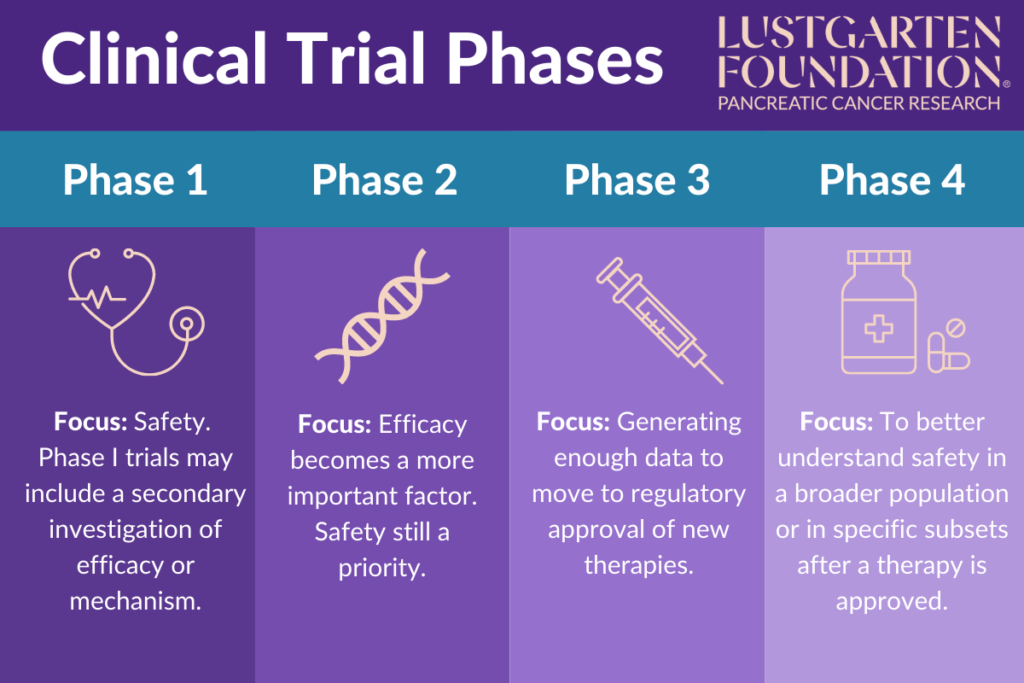
Clinical Trial Phases The master of public health (mph), is a swiss degree awarded by james lind institute, switzerland. james lind institute is an approved post secondary higher educational institution with the authority to award private degrees in switzerland. the institute is registered in the canton of geneva, switzerland under the uid che 255.747.977. In may 1750 he was elected a fellow of the royal college of physicians of edinburgh (rcpe minutes 1.iv 1750). subsequent minutes of the college show that he attended meetings regularly and the 1756 minutes record lind’s appointment as college treasurer (rcpe minutes 2.xii.1756). he was also the college’s representative on the board of the.

Comments are closed.