Cooling Curve Eutectic Phase Diagram Practice Problems

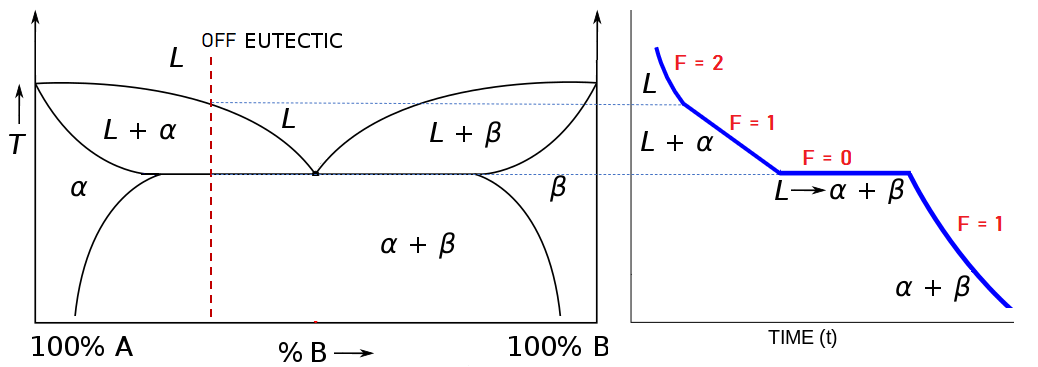
Heating And Cooling Curves вђ Overview Examples Expii By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 12.5: interpretation of cooling curves is shared under a cc by nc sa. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling.
Cooling Curve Eutectic Phase Diagram Practice Problems вђ Otosection 139. bi mass fraction of sn sn. 3 dimensional depiction of temperature composition phase diagram of bismuth, tin, and lead at 1atm. the diagram has been simplified by omission of the regions of solid solubility. each face of the triangular prism is a two component temperature composition phase diagram with a eutectic. Problem #3 the cs k phase diagram is given on the next page. refer to it in answering the following questions. (a) for a sample of composition 20 at. % cs and 80 wt. % k held at 10°c, determine (i) the composition of the solid phase present at equilibrium (ii) the composition of the liquid phase present at equilibrium. Session #34: homework problems. for the binary system cu ni the following data are available from cooling experiments: (a) from these data and information provided in the periodic table, construct the phase diagram (t vs c). (b) at each of the following (t,c) coordinates, i.e., combinations of temperature and composition, what are the phases. 9.34 consider the hypothetical eutectic phase diagram for metals a and b, which is similar to that for the lead tin system, figure 9.8. assume that (1) α and β phases exist at the a and b extremities of the phase diagram, respectively; (2) the eutectic composition is 47 wt% b 53 wt% a; and (3) the composition of the β phase at the eutectic.

Cooling Curve Eutectic Phase Diagram Practice Problems вђ Switzerlandersing Session #34: homework problems. for the binary system cu ni the following data are available from cooling experiments: (a) from these data and information provided in the periodic table, construct the phase diagram (t vs c). (b) at each of the following (t,c) coordinates, i.e., combinations of temperature and composition, what are the phases. 9.34 consider the hypothetical eutectic phase diagram for metals a and b, which is similar to that for the lead tin system, figure 9.8. assume that (1) α and β phases exist at the a and b extremities of the phase diagram, respectively; (2) the eutectic composition is 47 wt% b 53 wt% a; and (3) the composition of the β phase at the eutectic. Figure 8.9.1 8.9. 1: phase diagram of a two component system that exhibits an eutectic point. the unlabeled regions on the sides of the diagram indicate regions where one solid is so miscible in the other, that only a single phase solid forms. this is different than the “two phase solid” region where there are two distinct phases, meaning. By removing the time axis from the curves and replacing it with composition, the cooling curves indicate the temperatures of the solidus and liquidus for a given composition. this allows the solidus and liquidus to be plotted to produce the phase diagram: this page titled 4.2.5: interpretation of cooling curves is shared under a cc by nc sa.

Comments are closed.