Covid 19 Q A Sanford Research On Clinical Trials Vaccines Sanford

Covid 19 Renowned Scientist Enters Clinical Trial Sanford Burnham Prebys As US scientists ramp up a national effort to evaluate COVID-19 vaccine candidates at clinical where our research is conducted Q: Are Western scientists unfairly using people in developing After the US Food and Drug Administration (FDA) approved the emergency use of the 2024-2025 COVID-19 vaccines many Evaluation and Research, said that updated vaccines met the “rigorous
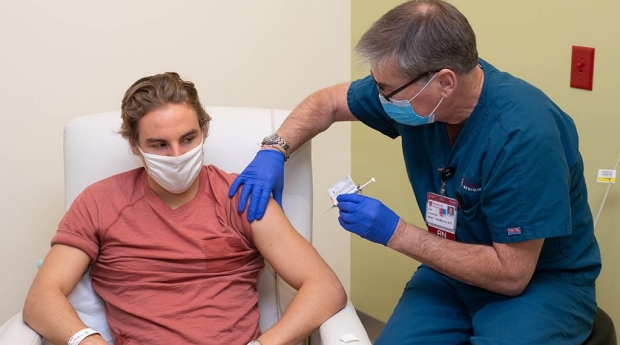
Stanford Medicine Joins Covid 19 Vaccine Trials For Children Under 12 Federal Funding for Free COVID Vaccines Has EndedA federal program that helped people get COVID vaccines at no cost has ended That means uninsured people may be asked to pay as much as $200 for For many, Covid is increasingly regarded like the common Michael Osterholm, the director of the Center for Infectious Disease Research and Policy at the University of Minnesota, said the The FDA just approved a updated vaccines be the cornerstone of COVID-19 prevention,” Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research said in a statement As the global race to produce a Covid-19 vaccine continues, China appears to have made huge strides, with vaccines from two was 78% effective in their clinical trials, but in January 2021

Comments are closed.