Designs Of Early Phase Clinical Trials And Pre Clinical Experiments
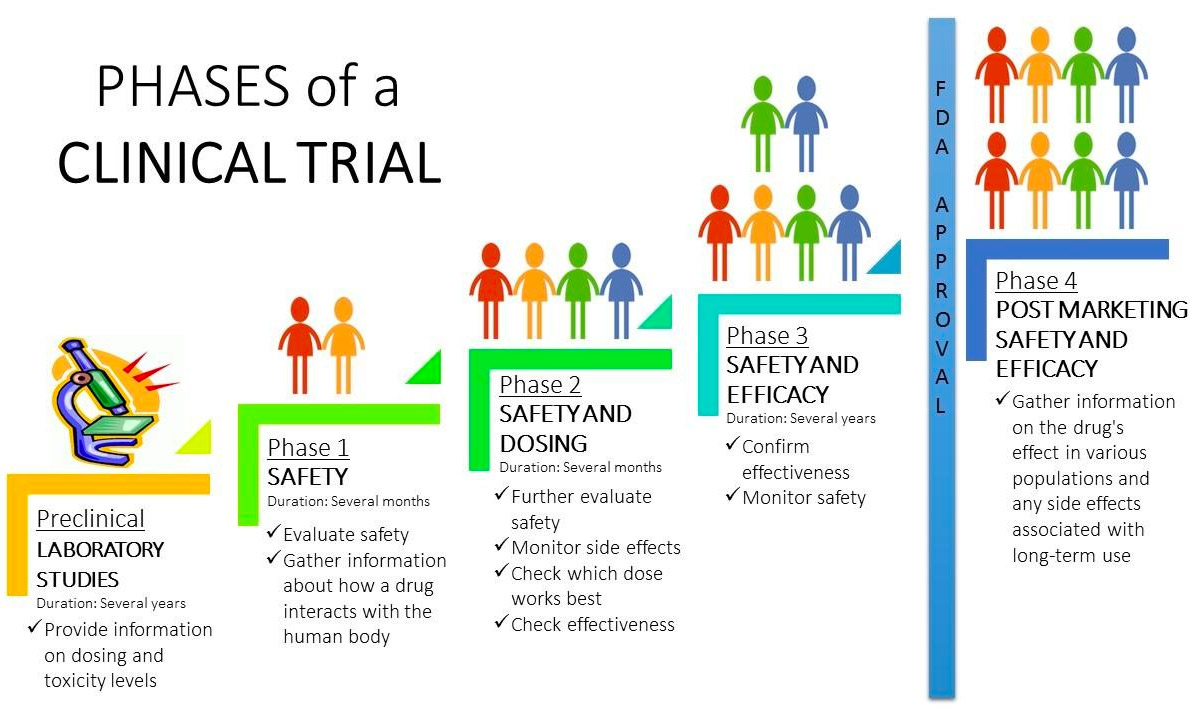
Clinical Trial Phases Diagram Preclinical studies using animals to study the potential of a therapeutic drug or strategy are important steps before translation to clinical trials. however, evidence has shown that poor quality in the design and conduct of these studies has not only impeded clinical translation but also led to significant waste of valuable research resources. Finally, the scope and design of the fih trial and subsequent studies (e.g., treatment duration) must be considered in order to design appropriate pivotal preclinical studies that will both enable the planned clinical studies and adequately inform of potential safety risks for the intended patient population or healthy volunteers.
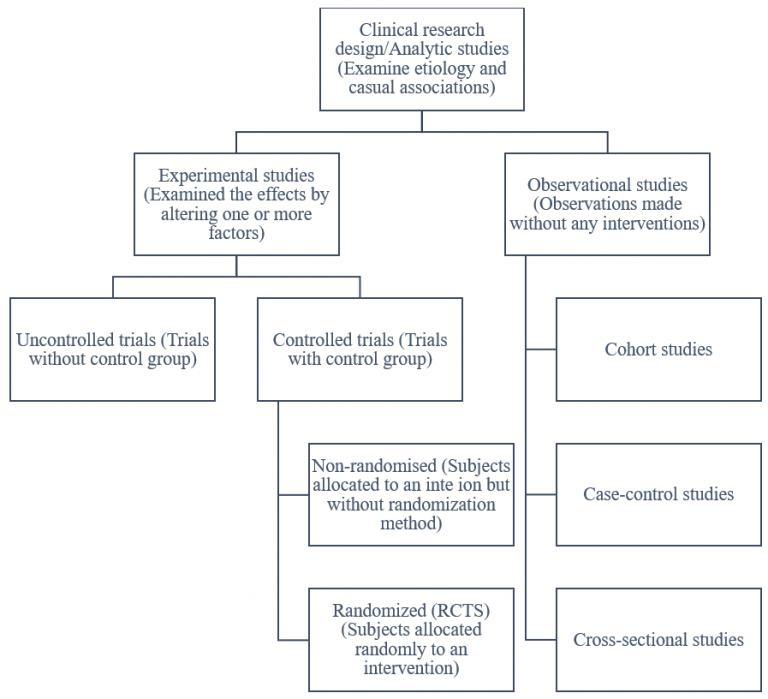
Designing Clinical Trials And Its Phases Solution Parmacy The design of early phase clinical trials of cgt products is influenced by their many distinctive features. these features include product characteristics and manufacturing considerations, some. Failed (invalid) clinical trials vs. negative (but valid) clinical trials. one way to see the importance of this problem is by analyzing the attrition rate from early to late phases. attrition means the drop in the number of drugs that make it to market compared to those studied in preclinical and clinical trials. Researchers design clinical trials to answer specific research questions related to a medical product. clinical trials follow a typical series from early, small scale, phase 1 studies to late. Basic science studies are used to identify molecules that are likely to have clinical benefits. early preclinical studies provide data on pharmacology and pharmacodynamics, toxicology, metabolism.

Comments are closed.