Different Phases Of Clinical Trials Drugs Testing And Development Of
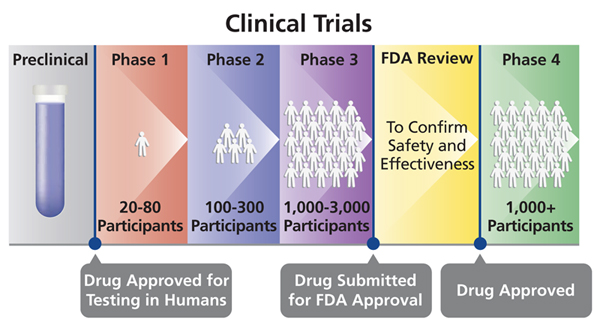
Different Phases Of Clinical Trials Drugs Testing And Development Of The drug development process. step 3: clinical research. while preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will. Phase 0 of a clinical trial is done with a very small number of people, usually fewer than 15. investigators use a very small dose of medication to make sure it isn’t harmful to humans before.
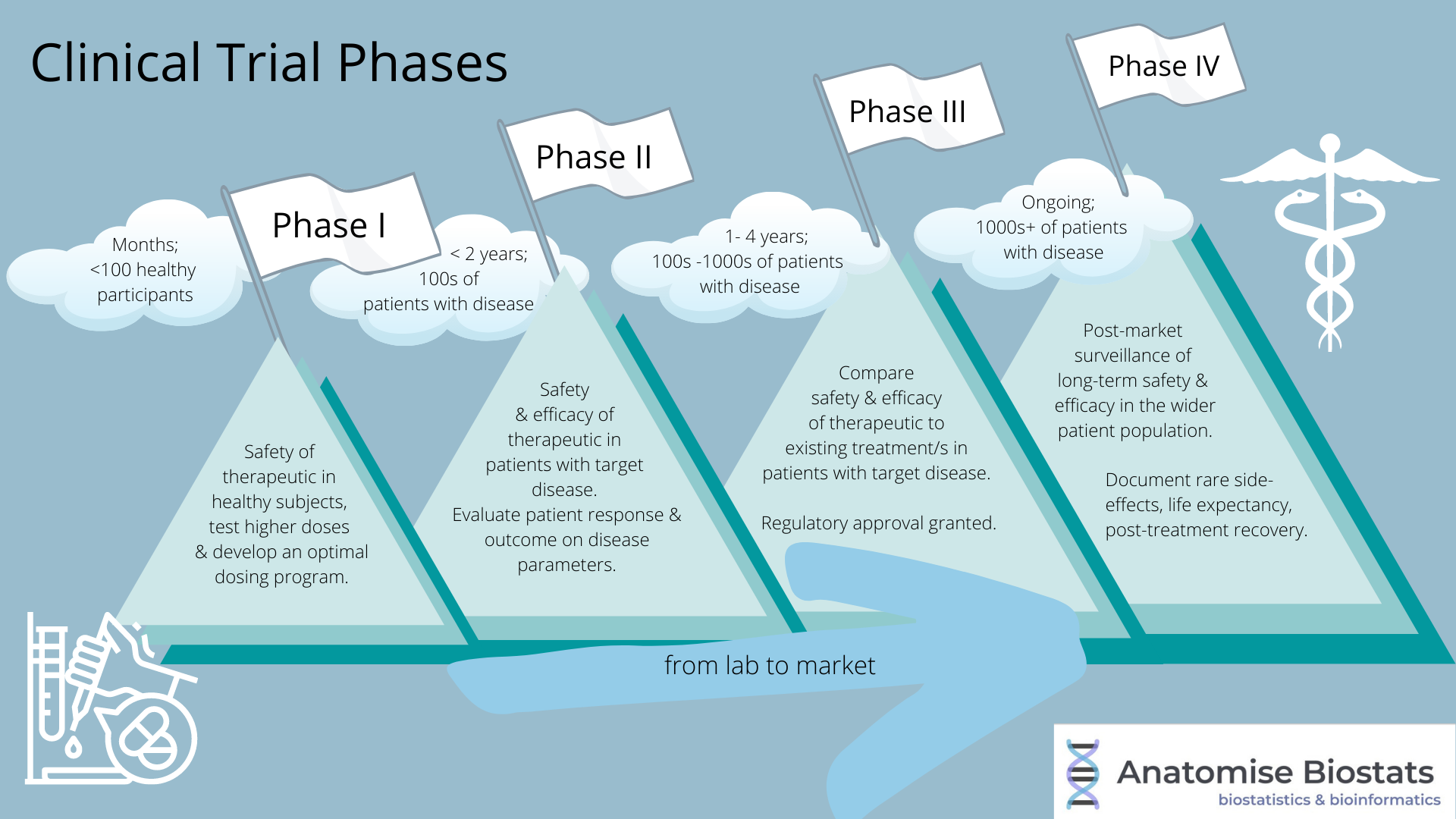
Clinical Trial Phases In Drug Development вђ Biostatistics The drug development process. drugs undergo laboratory and animal testing to answer basic questions about safety. clinical research drugs are tested on people to make sure they are safe. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. In fact, the overall probability of success for new molecular entities is only 12%. 2. to be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) fda review, and 5) safety monitoring. below, we explore each step in more detail. The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. [1] for drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study.
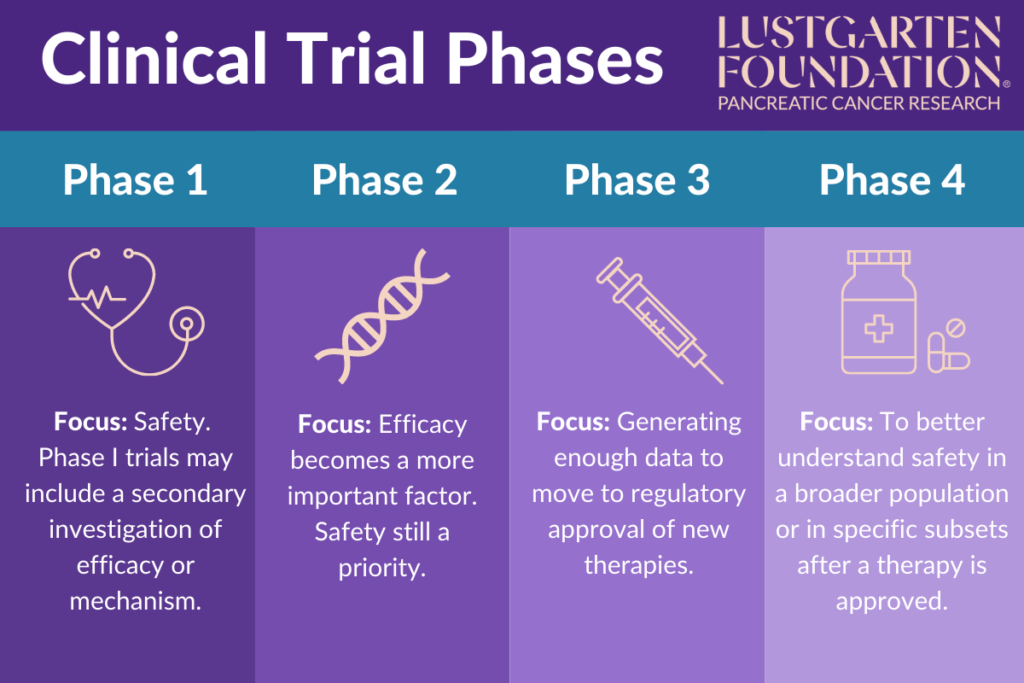
Clinical Trial Phases In fact, the overall probability of success for new molecular entities is only 12%. 2. to be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) fda review, and 5) safety monitoring. below, we explore each step in more detail. The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. [1] for drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study. Clinical trials are conducted for many reasons: to determine whether a new drug or device is safe and effective for people to use. to study different ways to use standard treatments or current. Drug trials are clinical research trials conducted to evaluate the safety and efficacy of various drugs in human subjects. the purpose of drug trials is to search for new and improved medications for the prevention and treatment of different medical conditions, as well as to examine known drugs that warrant further study and comparison.[1] blinding, randomization, adequate power, and a.

Comments are closed.