Drug Trials Testing Drugs On The Most Vulnerable
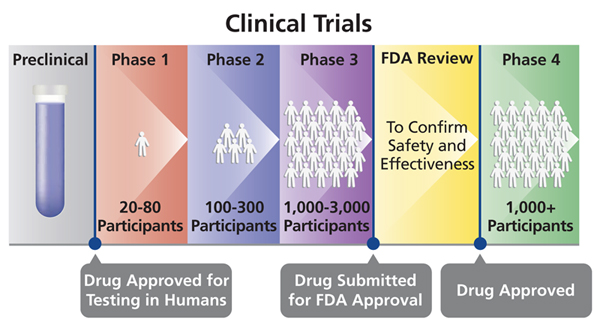
Different Phases Of Clinical Trials Drugs Testing And Development Of January 18, 2013: 16x9 investigates disturbing claims the pharmaceutical industry is testing drugs on the world's most vulnerable people without their consen. Clinical trials are a necessary step in drug development and are conducted throughout the world, both in developed and in developing countries. trials themselves are thus not per se immoral, and there are a variety of reasons to conduct responsible clinical trials in lmic. doing so, for example, is often the only way to test drugs and vaccines.

Drug Trials Free Of Charge Creative Commons Medical 5 Image For decades, women were often excluded from clinical drug trials. this was based in part on the unsubstantiated belief that fluctuations in female hormones would make women difficult to study. Sometimes, phase 4 trials are conducted after a product is already approved and on the market to find out more about the treatment's long term risks, benefits, and optimal use, or to test the. Antidepressant drug trials typically exclude those who need help most. now regulators and some researchers are asking if there needs to be a change in how these drugs are tested. Phase 1 studies are usually conducted in healthy volunteers. the goal here is to determine what the drug's most frequent side effects are and, often, how the drug is metabolized and excreted. the.
Principles Of Drug Abuse Treatment For Adolescents Summary National Antidepressant drug trials typically exclude those who need help most. now regulators and some researchers are asking if there needs to be a change in how these drugs are tested. Phase 1 studies are usually conducted in healthy volunteers. the goal here is to determine what the drug's most frequent side effects are and, often, how the drug is metabolized and excreted. the. Historical perspective. opinions and actions concerning women’s participation in clinical trials in the united states (u.s.) have changed through the years as governmental groups and researchers have best sought to protect the public’s health, but also try to better understand how women respond to prescription drugs (table 1).9 although there is recognition today of the need to include. It is important to test drugs and medical products in the people they are meant to help. it is also important to conduct research in a variety of people, because different people may respond.

Comments are closed.