First Line Immunotherapy Impower133 Regimen
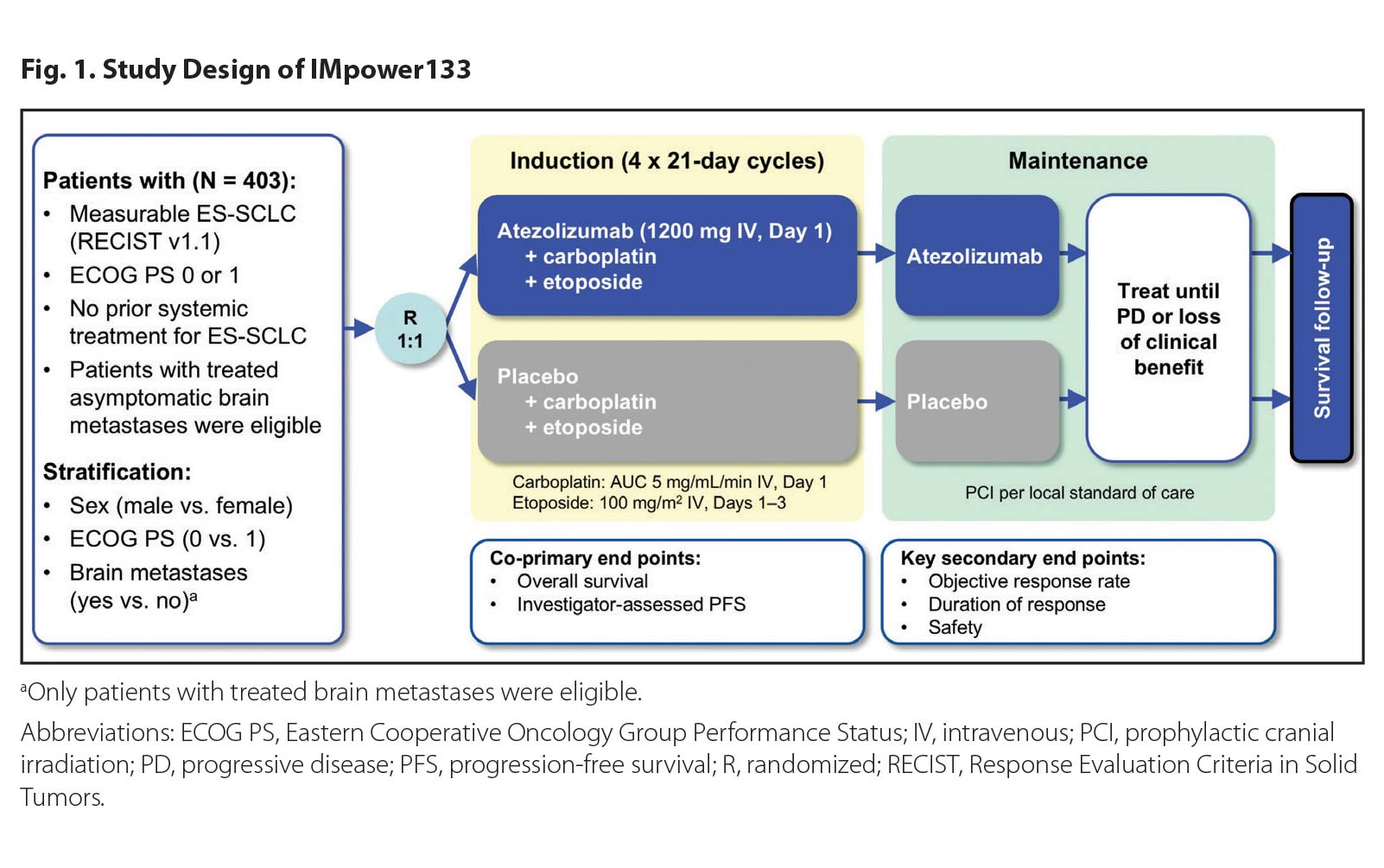
Impower133 Finally Moving The Needle In Sclc Ilcn Org Ilcn Wclc The impower133 trial evaluated the efficacy and safety of adding atezolizumab or placebo to first line treatment with carboplatin and etoposide in patients with extensive stage small cell lung cancer. First line immunotherapy: impower133 regimen. transcript: naiyer a. rizvi, md: we’ve clearly moved forward with immunotherapy in the second and third line settings. but, again, from the first.

First Line Immunotherapy Impower133 Regimen Youtube Purpose: impower133 (clinicaltrials.gov identifier: nct02763579), a randomized, double blind, phase i iii study, demonstrated that adding atezolizumab (anti programmed death ligand 1 [pd l1]) to carboplatin plus etoposide (cp et) for first line (1l) treatment of extensive stage small cell lung cancer (es sclc) resulted in significant improvement in overall survival (os) and progression free. Recently, immunotherapy has gained approval as a first line treatment for metastatic sclc in combination with chemotherapy. it has also been sanctioned as a third line treatment for metastatic sclc following the unsuccessful outcomes of two prior chemotherapy regimens [ 16 ]. An updated analysis (median survival follow up, 22.9 months) further confirmed atezolizumab plus cp et as a new standard of care for first line treatment of es sclc [6]. although icis plus chemotherapy have improved survival in the first line es sclc setting [1], long term survival and safety data are lacking. at the time of impower133 study. Conclusions: the addition of atezolizumab to chemotherapy in the first line treatment of extensive stage small cell lung cancer resulted in significantly longer overall survival and progression free survival than chemotherapy alone. (funded by f. hoffmann la roche genentech; impower133 clinicaltrials.gov number, nct02763579 .).

Comments are closed.