Free Mini Course Eu Mdr 2017 745 Medical Device Regulation

Free Mini Course Eu Mdr 2017 745 Medical Deviceођ This free online course explains the essentials of european medical device regulations (eu mdr) 2017 745. the course provides a way for learners to study the complex and detailed regulations, including the improvements in the eu mdr, the technical files elements, post market responsibilities, increased requirements related to market actors. Free medical device regulation online training course (eu mdr 2017 745) description. participate to a 6 days email course to learn more on the medical device regulation mdr 2017 745. during those 6 days, you will learn key information. during the last day, you will receive a link for a quizz.

Medical Device Regulation Mdr 2017 745 Course And Certific Mdr training resources. – regulation 745 2017 on medical devices: main changes and timeline to implementation. – regulation 745 2017 on medical devices: obligations of the economic operators, from eudamed registration to responsible person. – regulation 745 2017 on medical devices: unique device identification. – regulation 745 2017 on. This is an excerpt from the course "introduction to the medical device regulation (eu) 2017 745" which is available at: medicaldevicehq medical. This course is broken into nine sections. the objective of the course is to explain the medical device regulation 2017 745 in simple terms: section 1 introduction. introduction to the medical device regulation 2017 745. why was there a change from the directive to the mdr 2017 745? the timelines for the transition of the mdr 2017 745. This online course focuses on the european regulation for medical devices, the mdr. it covers an orientation of the medical device regulation according to (eu) 2017 745 as well as related guidance, like mdcg, and how to apply to a notified body for conformity assessment. topics covered include: how to work with the mdr, obligations of the.

Free Mini Course Eu Mdr 2017 745 Medical Deviceођ This course is broken into nine sections. the objective of the course is to explain the medical device regulation 2017 745 in simple terms: section 1 introduction. introduction to the medical device regulation 2017 745. why was there a change from the directive to the mdr 2017 745? the timelines for the transition of the mdr 2017 745. This online course focuses on the european regulation for medical devices, the mdr. it covers an orientation of the medical device regulation according to (eu) 2017 745 as well as related guidance, like mdcg, and how to apply to a notified body for conformity assessment. topics covered include: how to work with the mdr, obligations of the. Recognize and interpret the key qms requirements of the eu mdr (2017 745) appreciate that the range of medical device classifications mean differing requirements in the context of auditing. plan for and conduct eu mdr (2017 745) qms audits to establish and maintain compliance against these requirements. report on any identified nonconformities. Eu medical device regulation (mdr) 2017 745 – qms auditor training course. level internal auditor duration 3 days available to book: virtual instructor led training £1885 vat book your place. the medical device regulation (mdr) is the legislation detailing the requirements that manufacturers must meet to place medical devices on the market.

Complete Guide Medical Device Classification Eu Mdr Free Pdf Recognize and interpret the key qms requirements of the eu mdr (2017 745) appreciate that the range of medical device classifications mean differing requirements in the context of auditing. plan for and conduct eu mdr (2017 745) qms audits to establish and maintain compliance against these requirements. report on any identified nonconformities. Eu medical device regulation (mdr) 2017 745 – qms auditor training course. level internal auditor duration 3 days available to book: virtual instructor led training £1885 vat book your place. the medical device regulation (mdr) is the legislation detailing the requirements that manufacturers must meet to place medical devices on the market.
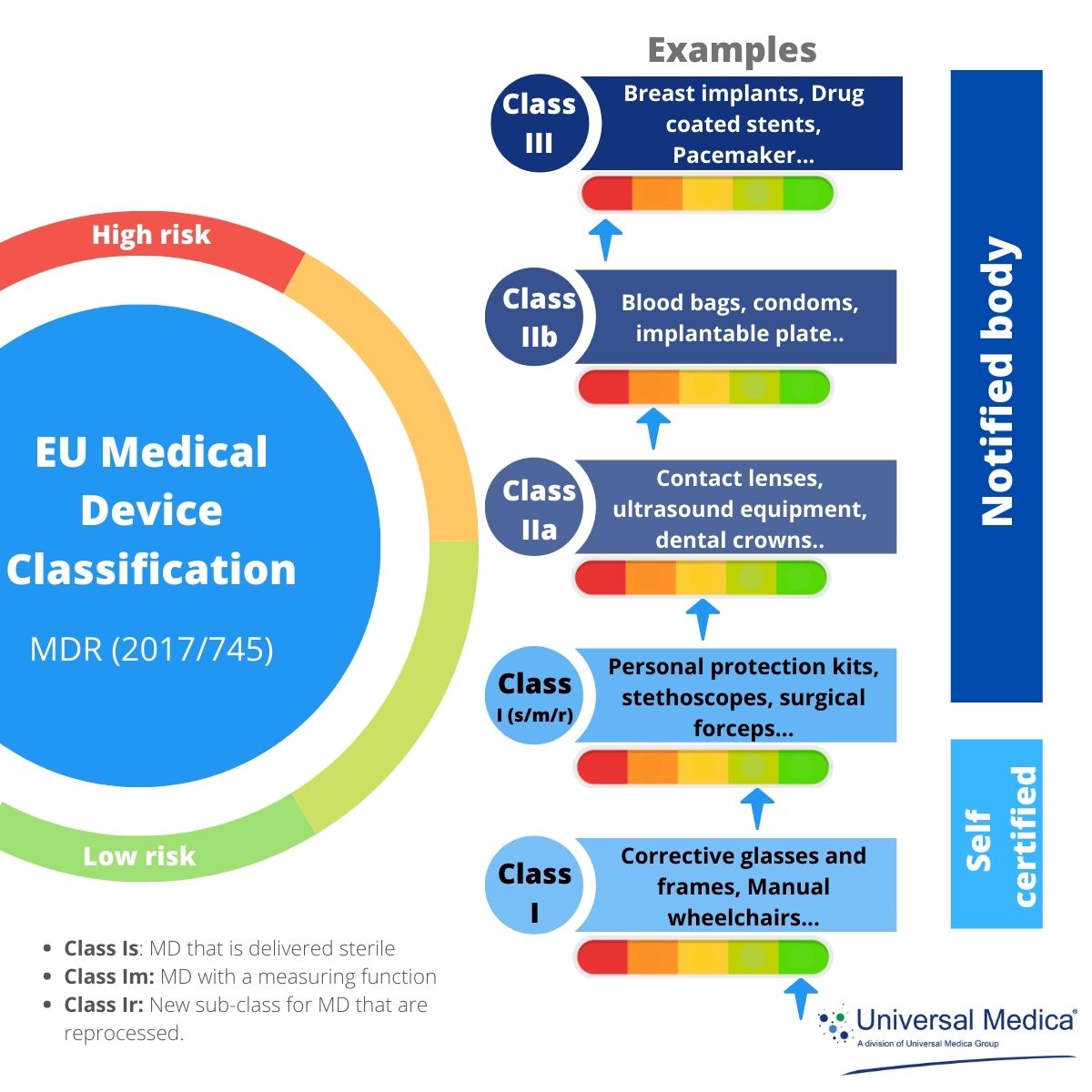
Key Aspects Of New Eu Medical Devices Regulation Eu 2017 745о

Comments are closed.