Heating Cooling Curve Calculations Eq Why Is An Ideal Heating

Heating Cooling Curve Calculations Eq Why Is An Ideal Heating The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. The energy change associated with each common phase change is shown in figure 2.5.1 2.5. 1. Δ h is positive for any transition from a more ordered to a less ordered state and negative for a transition from a less ordered to a more ordered state. previously, we defined the enthalpy changes associated with various chemical and physical processes.
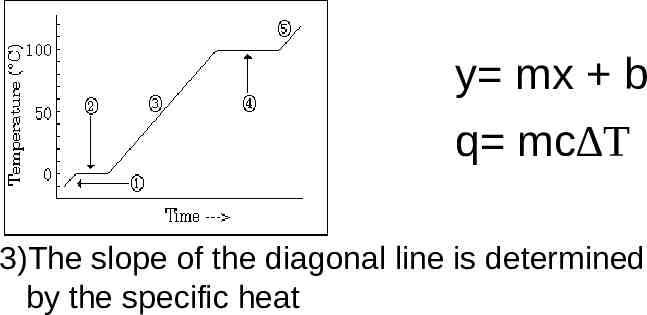
Heating Cooling Curve Calculations Eq Why Is An Ideal Heating Figure \(\pageindex{1}\): a typical heating curve for a substance depicts changes in temperature that result as the substance absorbs increasing amounts of heat. plateaus in the curve (regions of constant temperature) are exhibited when the substance undergoes phase transitions. consider the example of heating a pot of water to boiling. Thermochemistry crash course on heating & cooling curves. explained and broken up into each component of phases and phase changes, explaining what equations. A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember. Ethyl chloride (c2h5cl) boils at 12 °c. when liquid c2h5cl under pressure is sprayed on a room temperature (25 °c) surface in air, the surface is cooled considerably. (b) assume that the heat lost by the surface is gained by ethyl chloride.

Heating Cooling Curve Calculations Eq Why Is An A quick note about cooling curves. let's say we wanted to go from steam to ice. we would use a cooling curve. the cooling curve is a mirror image of the heating curve. so, it will start at a high temperature and have downward diagonals. the diagonals alternate with plateaus. the flat lines are the enthalpy of condensation and freezing. remember. Ethyl chloride (c2h5cl) boils at 12 °c. when liquid c2h5cl under pressure is sprayed on a room temperature (25 °c) surface in air, the surface is cooled considerably. (b) assume that the heat lost by the surface is gained by ethyl chloride. Heating curves tutorial: how to calculate enthalpy changes in heating & cooling | crash chemistry. Understanding heating and cooling curves is crucial for grasping how substances absorb or release heat during phase changes. as a substance heats up, it undergoes an endothermic process, indicated by a positive heat variable (q), absorbing energy to break molecular bonds and transition from solid to liquid (melting or fusion) and eventually to gas (vaporization).

Heating And Cooling Curves вђ Overview Examples Expii Heating curves tutorial: how to calculate enthalpy changes in heating & cooling | crash chemistry. Understanding heating and cooling curves is crucial for grasping how substances absorb or release heat during phase changes. as a substance heats up, it undergoes an endothermic process, indicated by a positive heat variable (q), absorbing energy to break molecular bonds and transition from solid to liquid (melting or fusion) and eventually to gas (vaporization).

Comments are closed.