How Does Air Pressure Affect The Boiling Point Of Water

Warm Up How Does Air Pressure Affect The Boiling Point Of Water Give As elevation increases, atmospheric pressure and boiling point decrease. boiling point is the point at which vapour pressure equals atmospheric pressure. in a liquid, some particles always have enough energy to escape to the gas phase. gaseous particles are also returning to the liquid. the vapour pressure is the pressure exerted by the gas when the amount of particles leaving the liquid. One formula for calculating the boiling point of water uses the known boiling point at sea level, 100°c, the atmospheric pressure at sea level and the atmospheric pressure at the time and elevation where the boiling takes place. the formula: bp {corr}=bp {obs} (p {obs} 760\text { mmhg})\times 0.045^o\text {c mmhg} bp corr = bp obs −(p.

Atmospheric Pressure And The Boiling Point Of Water Students 1 answer. the boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. raising the atmospheric pressure will raise the boiling point. conversely, lowering the atmospheric pressure will lower the boiling point of the liquid. this phenomena is due to the fact that the vapor pressure of water is temperature. Boiling. boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. the change from a liquid phase to a gaseous phase occurs when the vapor pressure of the liquid is equal to the atmospheric pressure exerted on the liquid. boiling is a physical change and molecules are not chemically altered during the. The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [ 1 ][ 2 ] and the liquid changes into a vapor. the boiling point of a liquid varies depending upon the surrounding environmental pressure. a liquid in a partial vacuum, i.e., under a lower pressure, has a lower. The boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea level atmospheric pressure (760 mm [29.92 inches] of mercury). at sea level, water boils at 100° c (212° f). at higher altitudes the temperature of the boiling point is.
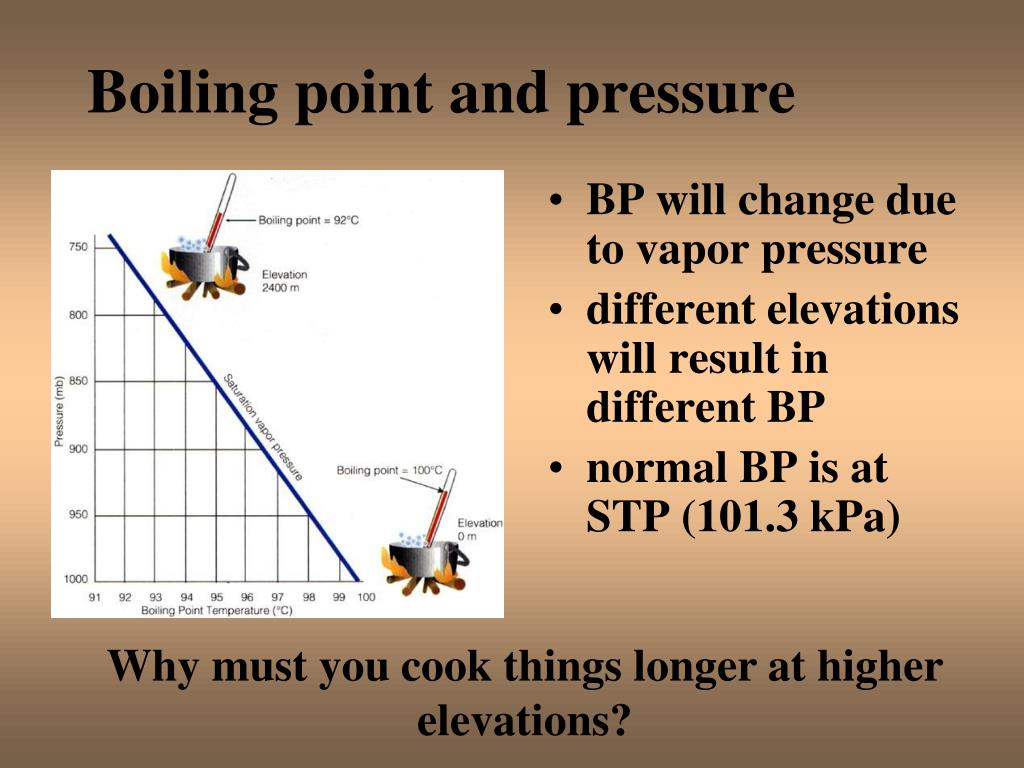
Warmup How Does Air Pressure Affect The Boiling Point Of The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid [ 1 ][ 2 ] and the liquid changes into a vapor. the boiling point of a liquid varies depending upon the surrounding environmental pressure. a liquid in a partial vacuum, i.e., under a lower pressure, has a lower. The boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea level atmospheric pressure (760 mm [29.92 inches] of mercury). at sea level, water boils at 100° c (212° f). at higher altitudes the temperature of the boiling point is. The boiling point is the temperature at which a liquid boils. the liquid changes into a vapor and the vapor pressure of the liquid is the same as the external environment. the simple definition of boiling point is that it is the temperature at which a liquid boils. for example, the boiling point of water at sea level is 100 °c or 212 °f. The normal boiling point of water is 100 °c or 212 °f. changes in elevation affect boiling point because they affect atmospheric pressure. the normal boiling point of water is 100 °c, 212 °f, or 373.1 k. the “normal” refers to sea level or an elevation of 0 meters or feet. but, the boiling point of water changes with elevation.

Comments are closed.