Influenza And The Dod
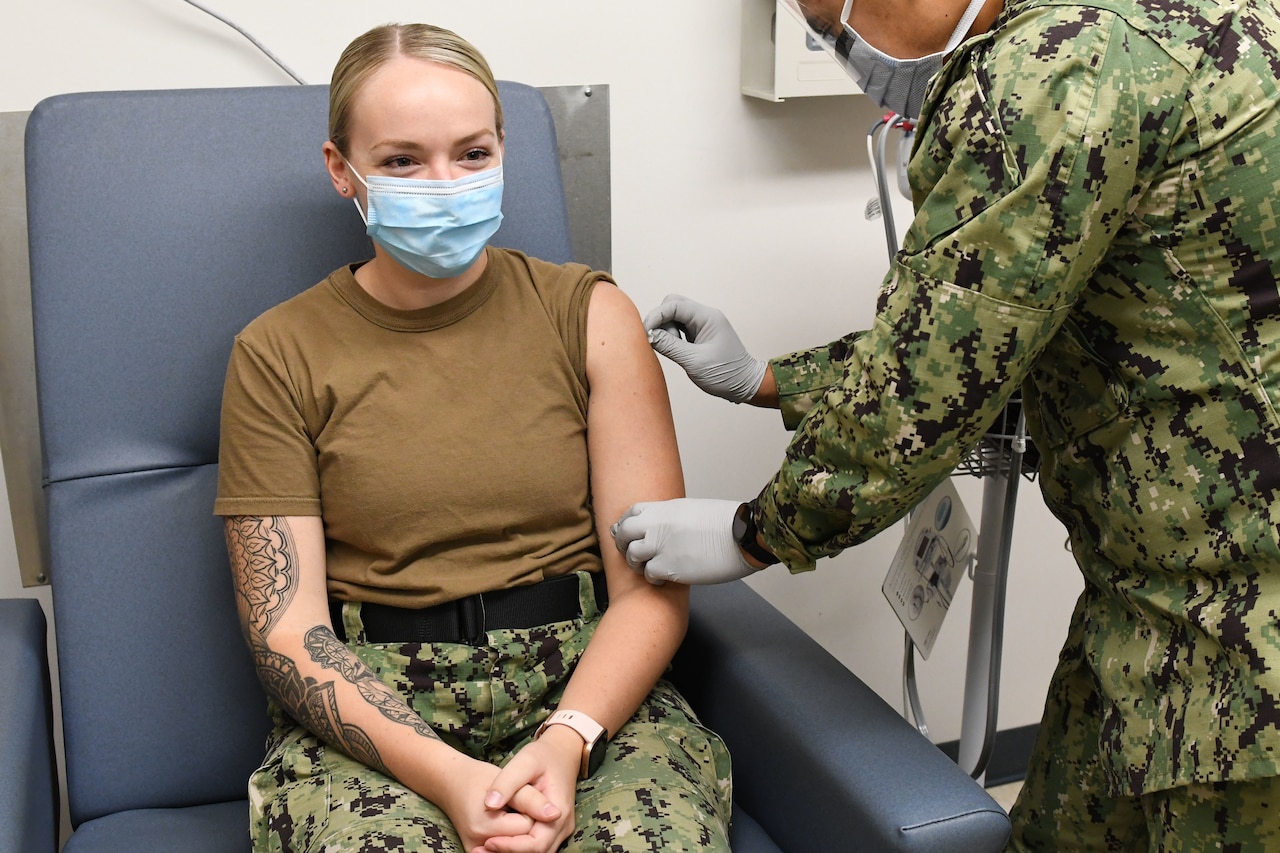
Flu Vaccine Essential During Covid 19 Pandemic U S Department Of Each march, the vaccines and related biological products advisory committee of the u.s. food and drug agency meets to review and discuss data on influenza strain circulation and vaccine effectiveness estimates for the current influenza season. 1 the committee then makes recommendations on the selection of strains for the influenza vaccines to be developed for the following influenza season. The 1918 spanish influenza pandemic spread within crowded military camps and traveled with soldiers, affecting 20% to 40% of u.s. army and navy personnel, disrupting training schedules and diverting resources away from combat support. 7 because influenza can spread quickly in the military, where soldiers are in close contact and mobile, the u.s. army requires soldiers to receive an annual flu.
Flu Cases Still High As First Human Universal Vaccine Trial Begins Cnn Summary of dod ve results. statistically significant ve estimates indicated an overall midseason ve of 54%. ve for influenza a 45%, indicating moderate protection. ve for influenza b ranged from 31 51%, indicating low to moderate protection. ve for a(h1) and ranged 42%, indicating moderate protection. The dod global emerging infections surveillance and response system (dod‐geis), a division of the armed forces health surveillance center (afhsc) since early 2008, centralized dod influenza and other respiratory disease surveillance efforts in 1998 and subsequently expanded worldwide surveillance and response initiatives starting in 2006. 4 , 10 , 11 the goal of the dod influenza. Pandemic influenza watchboard. as a key component of the department of defense's (dod's) pandemic influenza mitigation efforts, the medical countermeasures (mcm) directorate develops clinical guidelines for vaccines, diagnostics and antiviral policies that ultimately serve to protect department wide military and civilian personnel and. Ref c is defense health agency (dha) procedural instruction 6025.34, guidance for the dod influenza vaccination program. ref d is maradmin 631 10, requirement to track and improve individual.

Influenza Safety Naval Postgraduate School Pandemic influenza watchboard. as a key component of the department of defense's (dod's) pandemic influenza mitigation efforts, the medical countermeasures (mcm) directorate develops clinical guidelines for vaccines, diagnostics and antiviral policies that ultimately serve to protect department wide military and civilian personnel and. Ref c is defense health agency (dha) procedural instruction 6025.34, guidance for the dod influenza vaccination program. ref d is maradmin 631 10, requirement to track and improve individual. Ref c is defense health agency (dha) procedural instruction 6025.34, guidance for the dod influenza vaccination program. ref d is maradmin 631 10, requirement to track and improve individual. Influenza vaccination and respiratory virus interference among department of defense personnel during the 2017 2018 influenza season vaccine . 2020 jan 10;38(2):350 354. doi: 10.1016 j.vaccine.2019.10.005.

Comments are closed.