Institutional Review Board Orientation Irb Submission Process Ppt
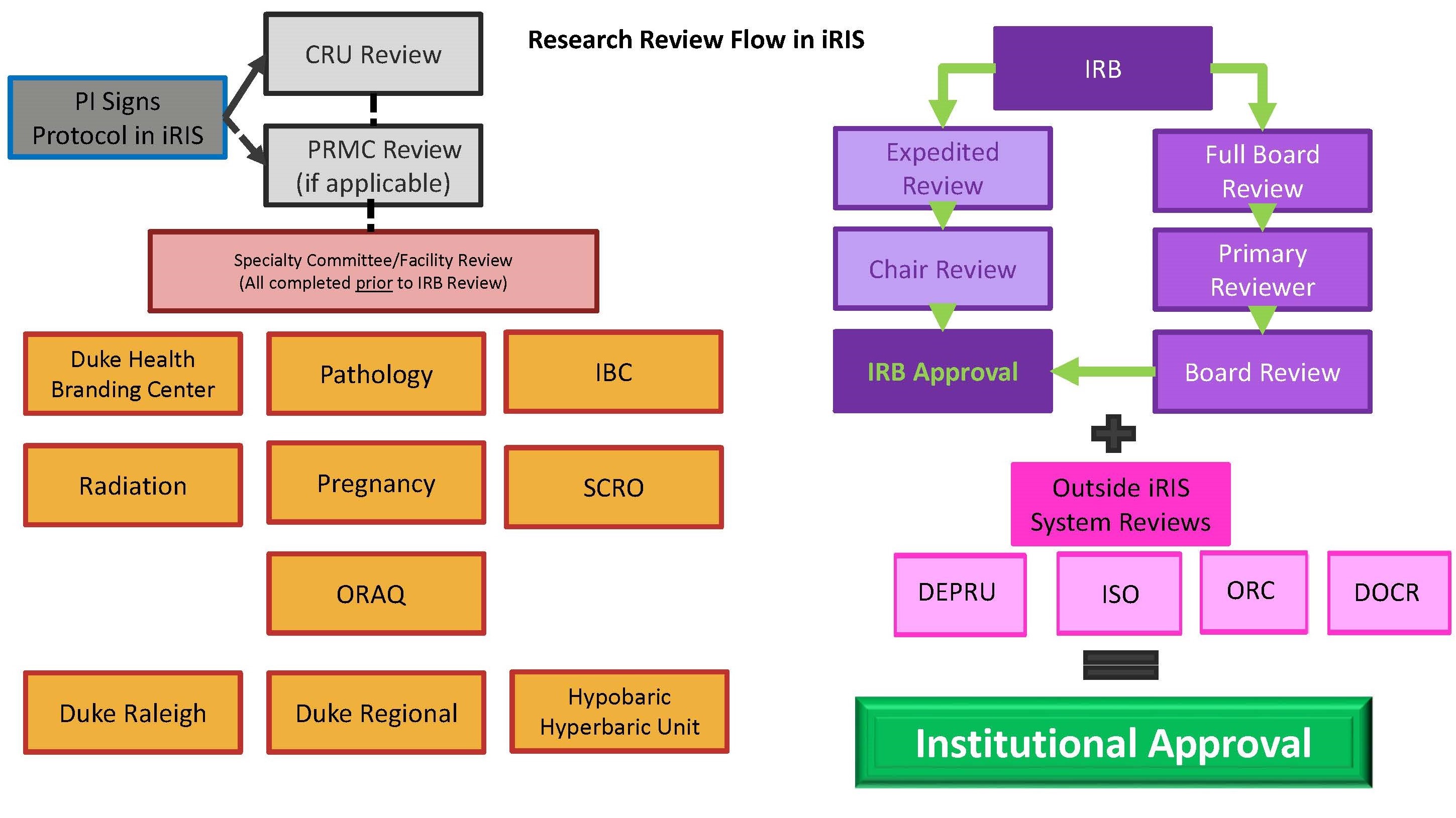
Irb Process Duke Health Institutional Review Board Single irb (sirb): federally funded multi site research commercial irb: sponsored research nci central irb: oncology group trials main site irb: institutional irb tribal irb: when required by (tribal) law, typically for research with a focus on american indians, alaskan natives tribes, or indigenous people. Presentation on theme: "institutional review board orientation & irb submission process"— presentation transcript: 1 institutional review board orientation & irb submission process 11 21 2012 man research protections and quality assurance institutional review board orientation & irb submission process kevin nellis, ms, cip executive director.

Ppt Institutional Review Board Irb Powerpoint Presentation Free The document discusses the role and responsibilities of an institutional review board independent ethics committee (irb iec). it states that an irb iec reviews clinical trial protocols to ensure the ethical treatment of study participants and protection of their rights and well being. the irb iec is composed of at least five members with. An institutional review board (irb), also known as an independent ethics committee (iec), is a committee responsible for reviewing and approving the ethical aspects of research involving human subjects. irbs iecs play a crucial role in protecting the rights, welfare, and safety of research participants. here are some key points about irbs iecs. An institutional review board (irb), also known as an independent ethics committee (iec), is a committee responsible for reviewing and approving the ethical aspects of research involving human subjects. irbs iecs play a crucial role in protecting the rights, welfare, and safety of research participants. here are some key points about irbs iecs. Here are some tips for completing the research protocol to ensure that the irb has the information it needs to review the study. keep in mind that the irb is reviewing the study to determine that it meets the criteria for approval. the more information the irb has, the easier it can be to make the required determinations. 1.

Institutional Review Board Orientation Irb Submission Process Ppt An institutional review board (irb), also known as an independent ethics committee (iec), is a committee responsible for reviewing and approving the ethical aspects of research involving human subjects. irbs iecs play a crucial role in protecting the rights, welfare, and safety of research participants. here are some key points about irbs iecs. Here are some tips for completing the research protocol to ensure that the irb has the information it needs to review the study. keep in mind that the irb is reviewing the study to determine that it meets the criteria for approval. the more information the irb has, the easier it can be to make the required determinations. 1. The irb process. the irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111 . federal regulation and institutional operating procedure require that the irb reviews all the research documents and activities that bear directly on the rights and welfare. The irb has the authority to: approve, require modifications in or disapprove all research activities. monitor research activities records (random audits) suspend or terminate any research (non compliance) the irb is now known as the arts & sciences irb, within office of research regulatory affairs (orra). located in downtown new brunswick.

Ppt Institutional Review Board Irb Powerpoint Presentation Id 690335 The irb process. the irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111 . federal regulation and institutional operating procedure require that the irb reviews all the research documents and activities that bear directly on the rights and welfare. The irb has the authority to: approve, require modifications in or disapprove all research activities. monitor research activities records (random audits) suspend or terminate any research (non compliance) the irb is now known as the arts & sciences irb, within office of research regulatory affairs (orra). located in downtown new brunswick.

Understanding The Institutional Review Board Process Ppt Download

Comments are closed.