Irb Process Pdf Institutional Review Board
Irb Process Duke Health Institutional Review Board The irb process. the irb reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 cfr 46.111 . federal regulation and institutional operating procedure require that the irb reviews all the research documents and activities that bear directly on the rights and welfare. An institutional review board (irb) is a committee set up by an organization to review, approve, and regulate research conducted by its members, on its premises, or under its sponsorship (babie, 2001). the national research act, passed by congress in 1974, directed all institutions receiving federal support for research and evaluation studies.

Irb Institutional Review Board Pdf Institutional Review ођ Institutional review board questions: contact the office of good clinical practice, 301 796 8340, or [email protected]. content current as of: 09 11 2019. under fda regulations, an. • the review of research involving human subjects by the institutional review board prior to the start of the research project. • seeking approval for making changes to the research protocol. • reporting to the irb any problems or adverse events related to the research project. • retaining copies of irb approval documents. The governing institutional review board (irb) for approval prior to beginning the research project. this article describes the irb process and offers tips for successful navigation of the procedure. a student’s guide to the irb: how to successfully navigate a potentially overwhelming process i did it! i got into graduate school. Irb, institutional policy or administrative practices, the number of irbs at the institution, affiliation with an institution, and local and state laws and regulations. to provide guidance on.

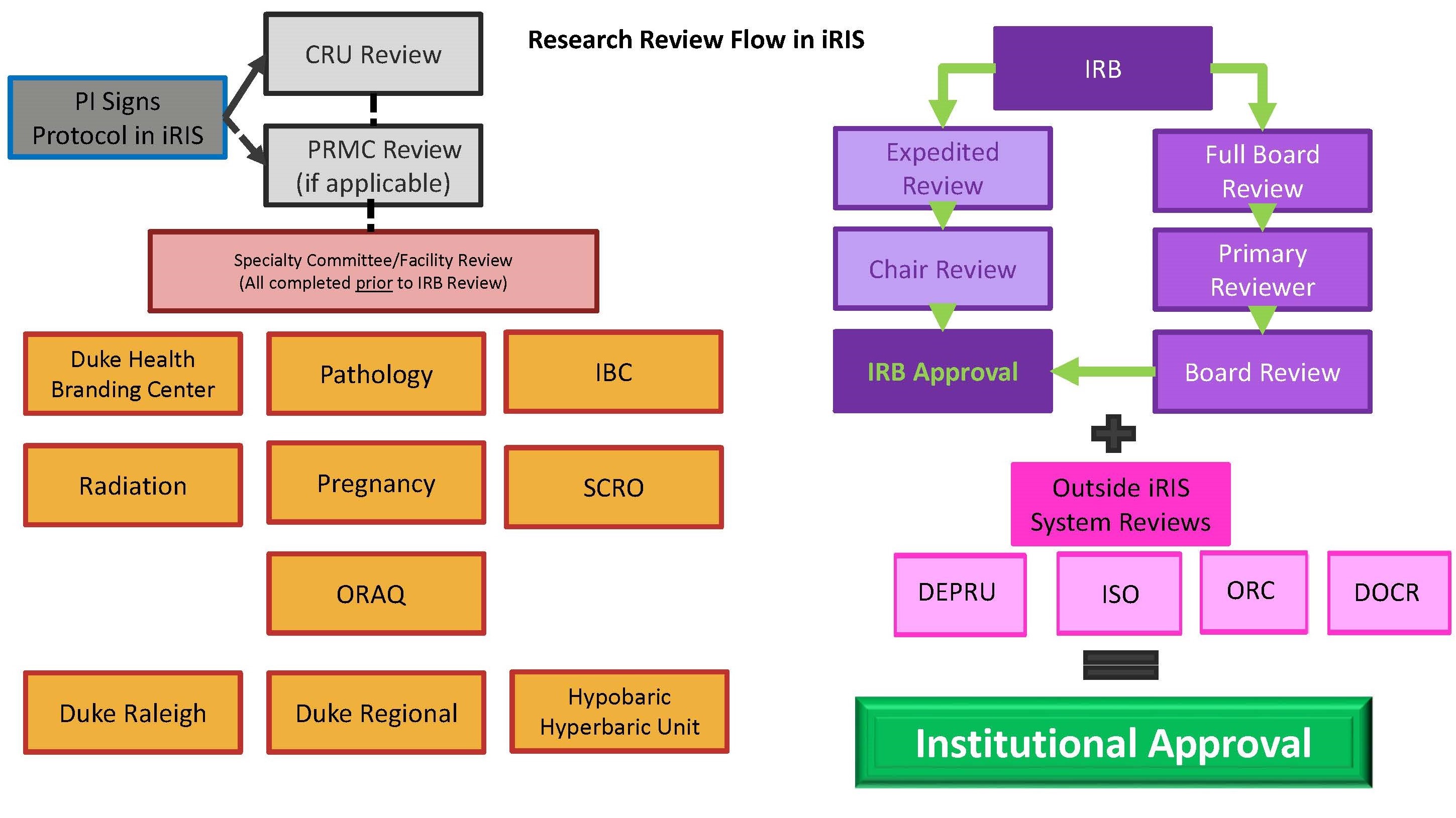
Flow Diagram Of Institutional Review Board Irb Processes Convened The governing institutional review board (irb) for approval prior to beginning the research project. this article describes the irb process and offers tips for successful navigation of the procedure. a student’s guide to the irb: how to successfully navigate a potentially overwhelming process i did it! i got into graduate school. Irb, institutional policy or administrative practices, the number of irbs at the institution, affiliation with an institution, and local and state laws and regulations. to provide guidance on. 1. introduction. the institutional review board (irb) is the focal point of oversight of research with human subjects in the us. 1 federal regulations require irb oversight for research with human subjects that is federally funded, is being submitted to the food and drug administration (fda) to support an application for a regulated product (such as an investigational drug or medical device. This guidance document is consistent with the goals of section 3023 of the cures act. this guidance is intended for institutions and institutional review boards (irbs) responsible for review and.

Institutional Review Board Application Sample And Approval Guide 1. introduction. the institutional review board (irb) is the focal point of oversight of research with human subjects in the us. 1 federal regulations require irb oversight for research with human subjects that is federally funded, is being submitted to the food and drug administration (fda) to support an application for a regulated product (such as an investigational drug or medical device. This guidance document is consistent with the goals of section 3023 of the cures act. this guidance is intended for institutions and institutional review boards (irbs) responsible for review and.

Comments are closed.