Medical Device Directive Mdd To Medical Device Regulation Mdr
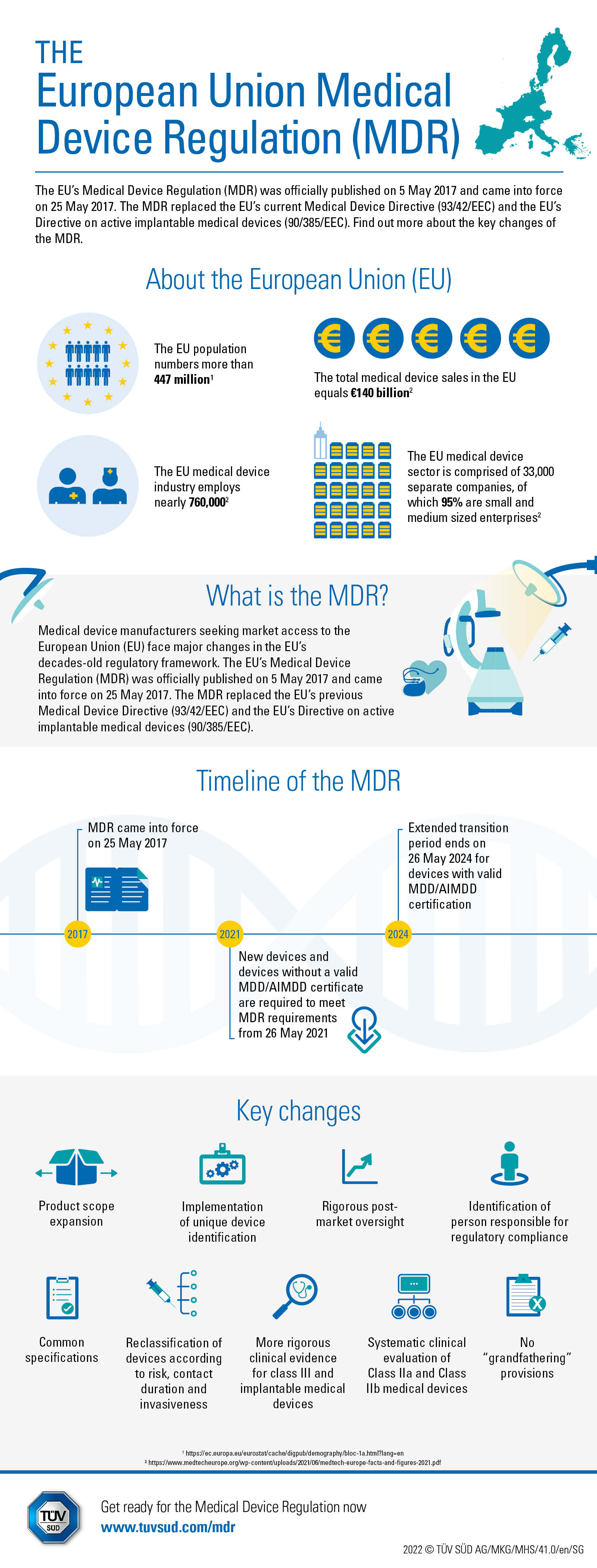
Infographic The Medical Device Regulation Tгњvеќ еѕ The medical device regulation (mdr) was established in 2007 and then revised for the first time in 2017. it is the successor of mdd and applies to manufacturers of medical devices sold or exported into europe, excluding products used inside the eu but may include those marketed outside it. We've highlighted these 13 points in text below as well. the new regulation is four times longer and contains five more annexes than its predecessor, the medical device directive (mdd). the word " safety " appears 290 times in the mdr. the mdd, by comparison, uses it only 40 times. significant changes in wording used in the new law will require.

Medical Device Regulation Mdr And Its Consequences For Union medical devices regulations – are you prepared? topic medical devices directive (93 42 eec), as amended medical devices regulation ((eu) 2017 745) comments the manufacturer has to draw up a declaration that the device conforms to the mdr and add a ce mark to the product. the declaration has to be kept up to date and available in the. Medical device regulation (mdr) the mdr, formally known as regulation (eu) 2017 745, came into effect on may 26, 2017, with a transition period that concluded on may 26, 2021. the mdr was introduced to address shortcomings in the mdd and to keep pace with technological advancements and increasing safety concerns. En. official journal of the european union. l 117 1. regulation (eu) 2017 745 of the european parliament and of the council. of 5 april 2017. on medical devices, amending directive 2001 83 ec, regulation (ec) no 178 2002 and regulation (ec) no 1223 2009 and repealing council directives 90 385 eec and 93 42 eec. Council directive 93 42 eec of 14 june 1993 concerning medical devices. council directive 93 42 eec of 14 june 1993 concerning medical devices. council directive 93 42 eec of 14 june 1993 concerning medical devices. oj l 169, 12.7.1993, p. 1–43 (es, da, de, el, en, fr, it, nl, pt) this document has been published in a special edition(s).

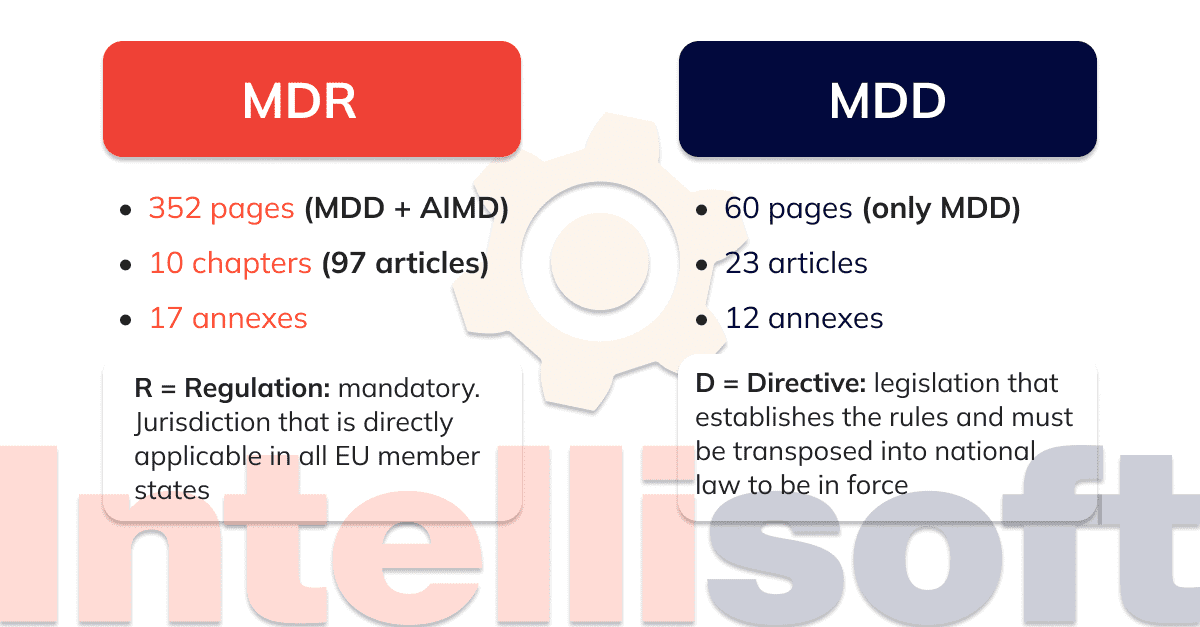
Medical Device Directive 93 42 Eec Mdd Directive Presentationeze En. official journal of the european union. l 117 1. regulation (eu) 2017 745 of the european parliament and of the council. of 5 april 2017. on medical devices, amending directive 2001 83 ec, regulation (ec) no 178 2002 and regulation (ec) no 1223 2009 and repealing council directives 90 385 eec and 93 42 eec. Council directive 93 42 eec of 14 june 1993 concerning medical devices. council directive 93 42 eec of 14 june 1993 concerning medical devices. council directive 93 42 eec of 14 june 1993 concerning medical devices. oj l 169, 12.7.1993, p. 1–43 (es, da, de, el, en, fr, it, nl, pt) this document has been published in a special edition(s). The mdr is significantly more comprehensive and detailed compared to the mdd. while the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. this table provides a comparison of some of the annexes of the mdd and mdr. the table is an excerpt from the mdr ivdr smart support available in. The medical device directive — council directive 93 42 eec of 14 june 1993 concerning medical devices —is intended to harmonise the laws relating to medical devices within the european union. the md directive is a 'new approach' directive and consequently in order for a manufacturer to legally place a medical device on the european market.

Statement Transition From The Mdd 93 42 Eec Directive To The Eu The mdr is significantly more comprehensive and detailed compared to the mdd. while the mdd comprises 23 articles and 12 annexes over 60 pages, the mdr has 123 articles and 17 annexes over 175 pages. this table provides a comparison of some of the annexes of the mdd and mdr. the table is an excerpt from the mdr ivdr smart support available in. The medical device directive — council directive 93 42 eec of 14 june 1993 concerning medical devices —is intended to harmonise the laws relating to medical devices within the european union. the md directive is a 'new approach' directive and consequently in order for a manufacturer to legally place a medical device on the european market.
Eu Mdr Mdd Key Differences Infographic 52 Off

Comments are closed.