Multistage Clinical Trial Phases With Cost Involved New ођ
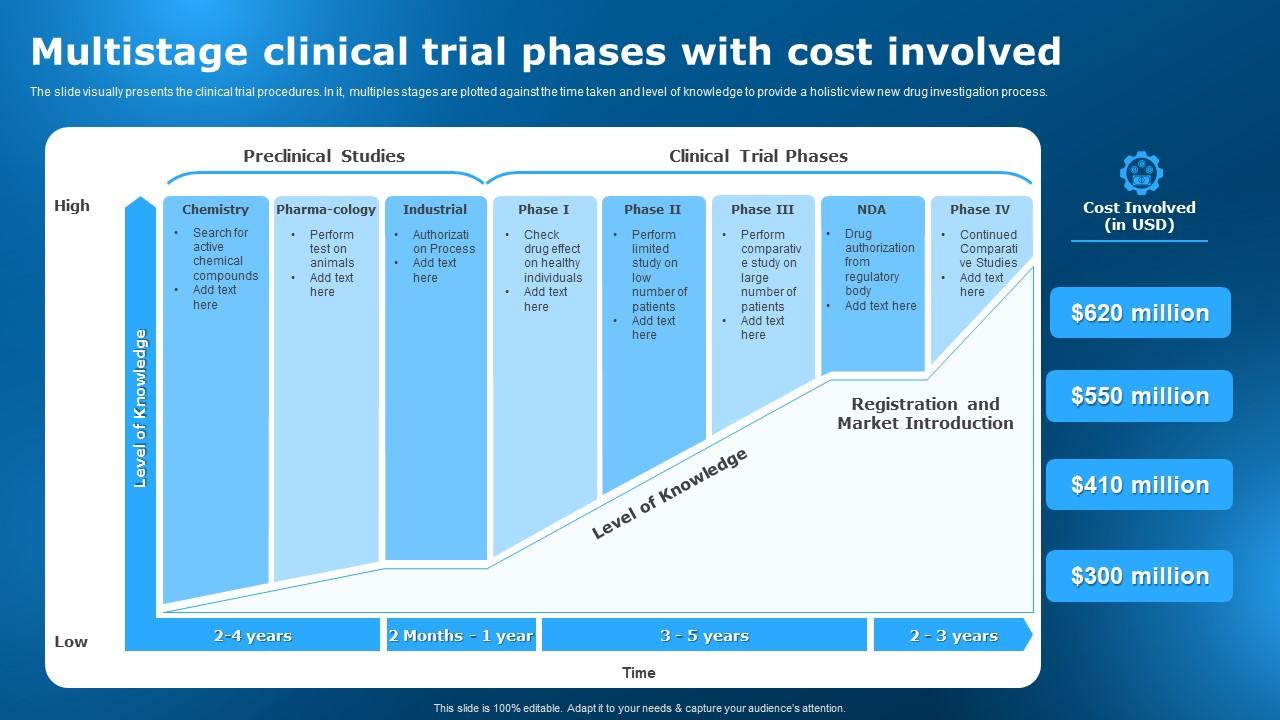
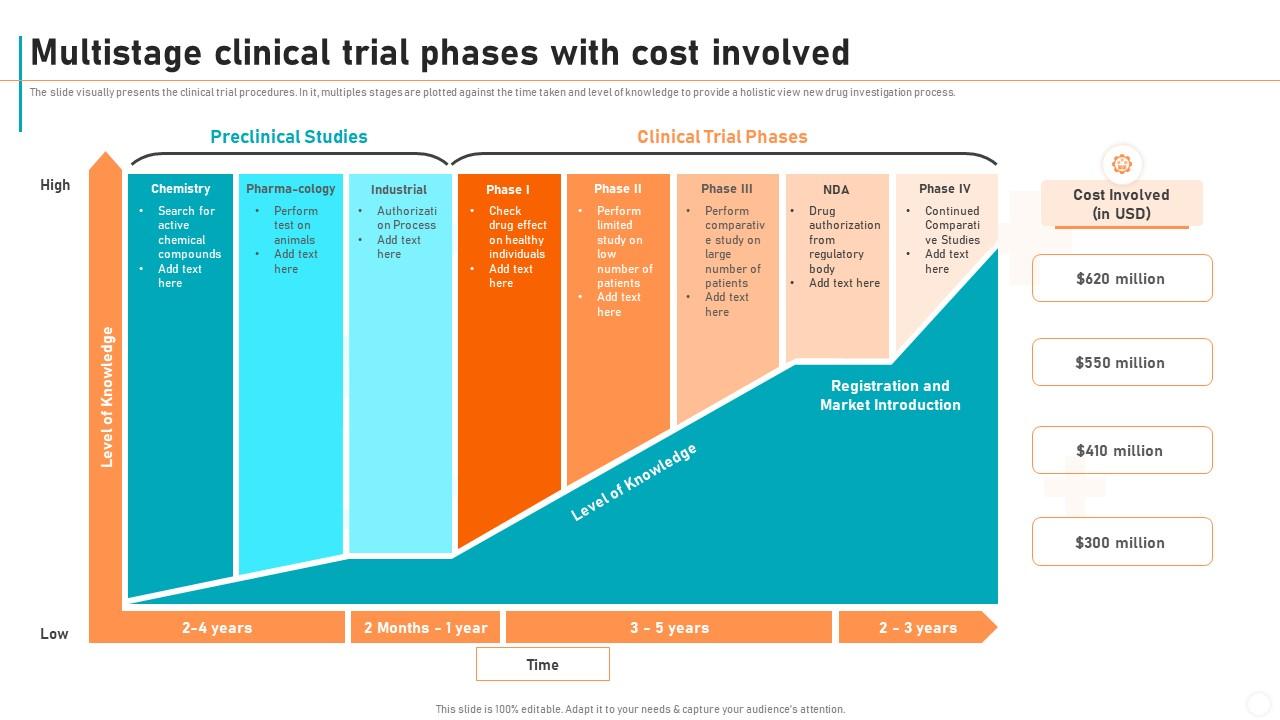
Multistage Clinical Trial Phases With Cost Involved Clinicalо Clinical trials to obtain fda approval typically account for small proportion of total drug research and development costs, study suggests. clinical trials that support fda approvals of new drugs have a median cost of $19 million, according to a new study by a team including researchers from johns hopkins bloomberg school of public health. Clinical trials can be separated into phase i (dose finding and safety), phase ii (activity or early efficacy), phase iii (efficacy compared with current standard of care) and occasionally phase iv (postmarketing studies). a new compound would usually have to go through phase i–iii studies sequentially with all of the financial and regulatory hurdles this poses. a recent study has estimated.
Multistage Clinical Trial Phases With Cost Involved New Dr Randomised controlled trials are becoming increasingly costly and time consuming. in 2011, royston and colleagues proposed a particular class of multi arm multi stage (mams) designs intended to speed up the evaluation of new treatments in phase ii and iii clinical trials. One such adaptive design is the mams trial. mams trials were first reported over 20 years ago as a way to accelerate the process of drug development.3. mams has been more commonly implemented in phase ii iii settings, although it can be applied in any trial phase. rather than a series of separate phase ii iii studies, mams trials aim to answer. Neither memantine nor trazodone improved efficacy outcomes compared with placebo. this result is sufficiently powered to warrant no further testing of trazodone or memantine in motor neuron disease at the doses evaluated in this study. the multiarm multistage design shows important benefits in reducing the time, cost, and participant numbers to reach a definitive result. Abstract. efficient clinical trial designs are needed to speed up the evaluation of new therapies. the multi arm multi stage (mams) randomized clinical trial designs have been proposed to achieve this goal. in this framework, multiple experimental treatments are compared against a common control arm in several stages.

Comments are closed.