Multistage Clinical Trial Phases With Cost Involved New Drug
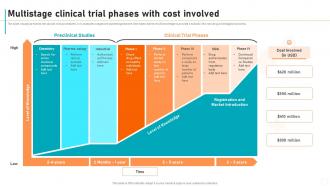
Multistage Clinical Trial Phases With Cost Involved New Drug Estimates of the total average capitalized (pre launch) r&d costs needed to bring a new compound to the market varied widely, from $161 million to $4.5 billion, depending on which research phases were included in the analysis (e.g. discovery and preclinical phases were not included in 13 estimations), the therapeutic class, the drug sample. The path to approval: clinical research phases. new treatments go through the following phases of clinical research: phase o trials. first in human clinical trial (optional) with 10 to 15 healthy volunteers where small amounts of the investigational drug are given to check if the drug behaves as expected in humans.

Multistage Clinical Trial Phases With Cost Involved New ођ Clinical trials to obtain fda approval typically account for small proportion of total drug research and development costs, study suggests. clinical trials that support fda approvals of new drugs have a median cost of $19 million, according to a new study by a team including researchers from johns hopkins bloomberg school of public health. As noted, the tufts study also factored in post approval development costs. in the new study by ismp, costs per drug varied substantially among pivotal clinical trials that would lead to marketing approval. overall, 45 of the 101 drugs analyzed were approved with a single trial, with a median cost of $28 million (iqr of $13 million to $62 million). In addition, 27 drugs were approved with 3–11 clinical trials each at an estimated overall median cost of us$91 million (iqr us$56 million–us$128 million) per drug. the reason for three or more trials was because approval was sought for several closely related indications, often combination or adjuvant therapy. Use of lower cost facilities and or inhome testing can reduce per trial costs by up to $0.8 million (up to 16 percent of cost per study) in phase 1, $4.3 million (up to 22 percent of cost per study) in phase 2, and $9.1 million (up to 17 percent of cost per study) in phase 3, depending on therapeutic area.
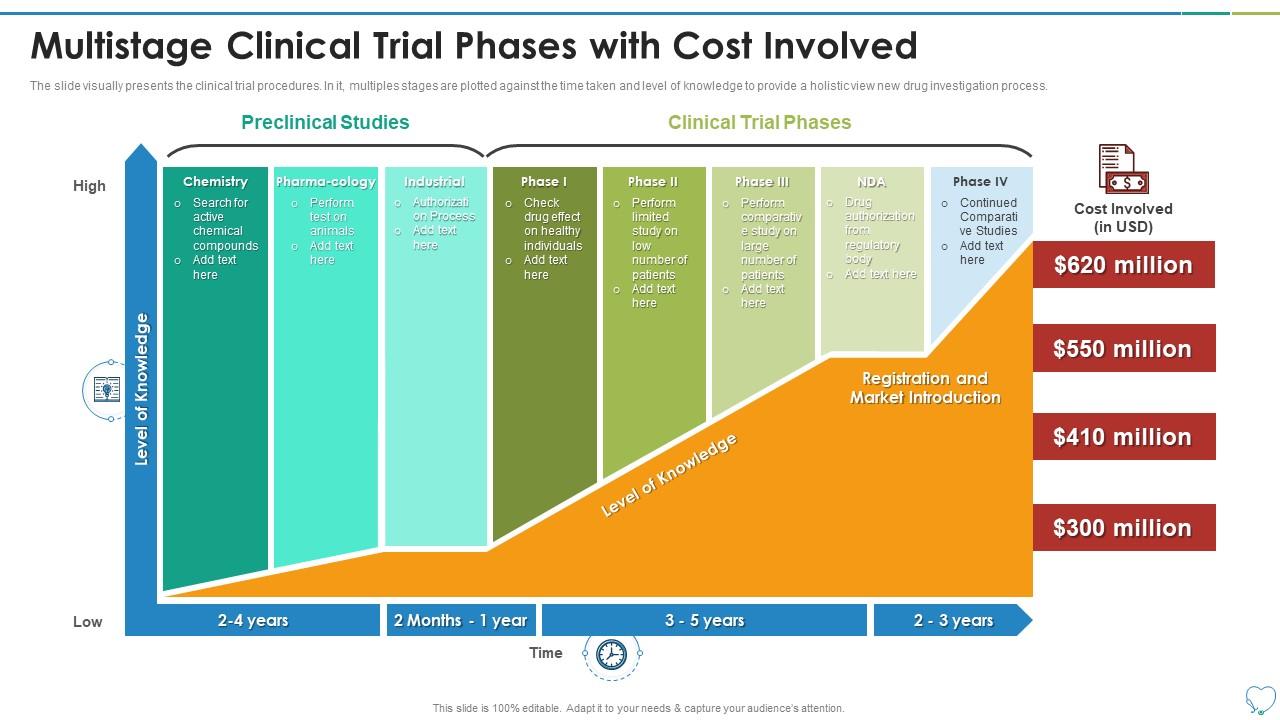
Multistage Clinical Trial Phases With Cost Involved Clinicalо In addition, 27 drugs were approved with 3–11 clinical trials each at an estimated overall median cost of us$91 million (iqr us$56 million–us$128 million) per drug. the reason for three or more trials was because approval was sought for several closely related indications, often combination or adjuvant therapy. Use of lower cost facilities and or inhome testing can reduce per trial costs by up to $0.8 million (up to 16 percent of cost per study) in phase 1, $4.3 million (up to 22 percent of cost per study) in phase 2, and $9.1 million (up to 17 percent of cost per study) in phase 3, depending on therapeutic area. Clinical trials can be separated into phase i (dose finding and safety), phase ii (activity or early efficacy), phase iii (efficacy compared with current standard of care) and occasionally phase iv (postmarketing studies). a new compound would usually have to go through phase i–iii studies sequentially with all of the financial and regulatory hurdles this poses. a recent study has estimated. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and.

Comments are closed.