Organic Chemistry Synthesis Of Aspirin Aspirin Physical Sciences

Organic Chemistry Synthesis Of Aspirin Pdf Aspirin Physical Experiment 614: synthesis of aspirin . section 1: purpose and summary . conduct a chemical reaction to produce aspirin. separate the aspirin from the reaction by products using vacuum filtration. analyze the aspirin and estimate its purity. acetylsalicylic acid, commonly known as aspirin, is the most widely used drug in the world today. James chickos, david garin, and val erian d'souza. university of missouri–st. louis; chemistry) 1: synthesis of aspirin (experiment) is shared under a license and was authored, remixed, and or curated by libretexts. analgesics are compounds used to reduce pain, antipyretics are compounds used to reduce fever. one popular drug that does both.
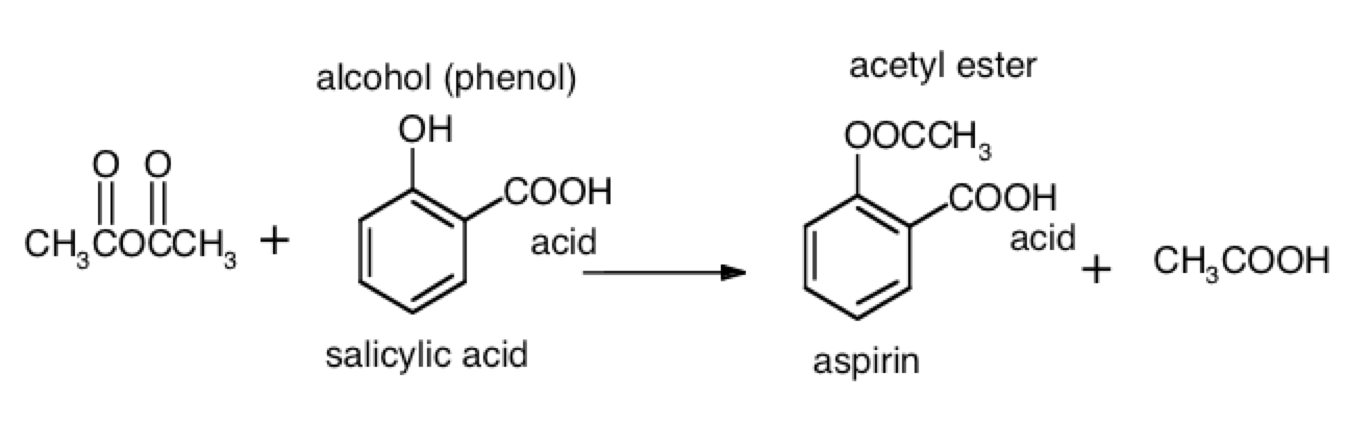
Aspirin The synthesis of aspirin is an organic chemistry experiment in many specifications for students of ages 16 18 years. each of the four levels take approximately 30 minutes to complete and are designed to be used as pre lab activities in class or as homework. these explanatory demonstration. this virtual experiment works best on a desktop pc. You will learn the different classes of organic compounds. you will then synthesize aspirin, nylon 6 10 and three different esters. safety glasses are required for this experiment. textbook reference: pp 516 517 . part 1: classes of organic compounds . several different organic compounds will be passed around the laboratory. gently waft each. Organic synthesis of aspirin from benzene. aspirin (acetylsalicylic acid) is a nonsteroidal anti inflammantory drug (nsaid) useful for alleviating pain, fever, and inflammation. in addition to being one of the most widely used drugs (44,000 tons consumed globally per year), it is also one of the earliest pharmaceuticals developed, being named. Acetylsalicylic acid is marketed under the name of aspirin for the home bayer being one of the drugs most consumed in the world. it was synthesized at the end of the last century by the german chemist felix hofmann. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic.
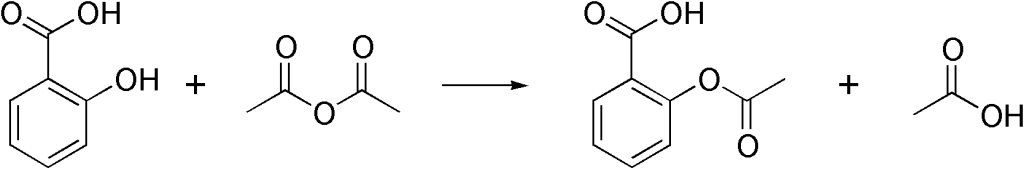
Organic Synthesis Of Aspirin Chemistry Formal Lab Writework Organic synthesis of aspirin from benzene. aspirin (acetylsalicylic acid) is a nonsteroidal anti inflammantory drug (nsaid) useful for alleviating pain, fever, and inflammation. in addition to being one of the most widely used drugs (44,000 tons consumed globally per year), it is also one of the earliest pharmaceuticals developed, being named. Acetylsalicylic acid is marketed under the name of aspirin for the home bayer being one of the drugs most consumed in the world. it was synthesized at the end of the last century by the german chemist felix hofmann. it uses a reaction of esterification catalyzed acid (h 2 so 4 or h 3 po 4 ), where the salicylic acid treated with acetic. Recrystallization of aspirin. place the remaining crude product into a clean 125 ml erlenmeyer flask. add ethanol (about 1 ml for every gram of crude product) and warm in the water bath at about 50°c. if lots of solid remains, add more ethanol in 1 ml portions. once most or all is dissolved, add 40 ml water. An experiment is described that is suitable for the early portion of the laboratory in a general chemistry course and integrates organic examples. it is the two step synthesis of aspirin starting from oil of wintergreen. the mechanism for this synthesis provides examples of three major classes of chemical reactions: hydrolysis, condensation, and proton transfer. to understand the chemistry.

Comments are closed.