Periodic Table With Electronegativity Values Labeled Image
:max_bytes(150000):strip_icc()/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png)
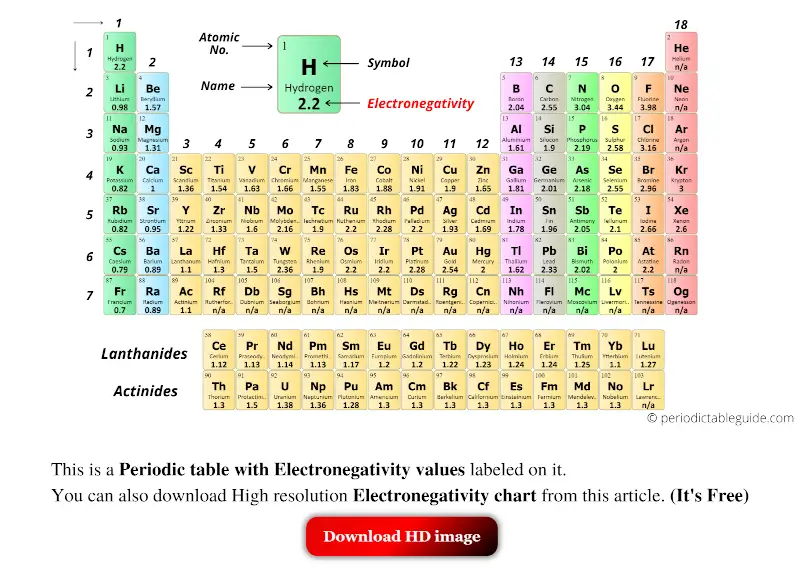
Printable Periodic Table Of The Elements Electronegativity The above image clearly shows you the periodic table with electronegativity values labeled on it. you can also get the hd printable periodic table with electronegativity, from this article only. Interactive periodic table showing names, electrons, and oxidation states. visualize trends, 3d orbitals, isotopes, and mix compounds. fully descriptive writeups.
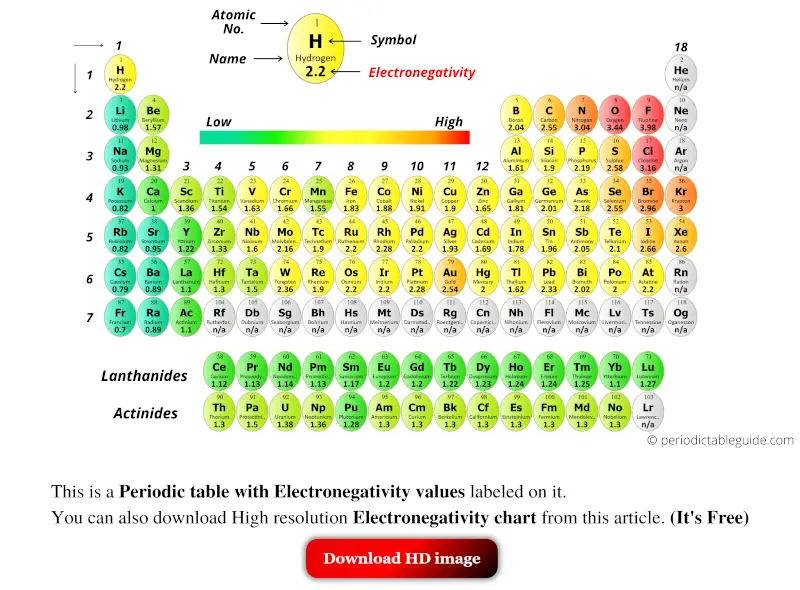
Periodic Table With Electronegativity Values Labeled Image Periodic table with electronegativity values (labeled image) june 18, 2023 by jay rana. electronegativity of all the elements of periodic table is mentioned in the above image. (note: electronegativity has no unit. a scale of electronegativity was designed by scientist linus pauling. this scale ranks the elements with respect to each other and. The periodic table of the elements (with electronegativities) 1 18 hydrogen 1 h 1.01 2.1 2 alkali metals alkaline earth metals transition metals lanthanides actinides other metals metalloids (semi metal) nonmetals 6.94 halogens noble gases element name 80 symbol beryllium electronegativity mercury hg 200.59 1.9 atomic # lithium avg. mass 13 14. On this scale, electronegativity is a dimensionless quantity and does not have any unit. this scale can be applied to determine the electronegativity values of the elements in the periodic table. typically, the values range from 0.7 to 4. the following image shows the periodic table of elements with the electronegativity values [1]. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots.

List Of Electronegativity Values Of The Elements On this scale, electronegativity is a dimensionless quantity and does not have any unit. this scale can be applied to determine the electronegativity values of the elements in the periodic table. typically, the values range from 0.7 to 4. the following image shows the periodic table of elements with the electronegativity values [1]. Explore how electronegativity changes with atomic number in the periodic table of elements via interactive plots. Electronegativity is the tendency of an atom to attract the electrons of another atom to form a bond. atoms with high electronegativity will strongly attract the electrons of other atoms. atoms with low electronegativity are more likely to have their bonding electrons pulled away. as you cross the periodic table from right to left. And this scale is known as pauling electronegativity scale.) the pauling electronegativity values of the element ranges from the most electronegative element (fluorine having electronegativity = 3.98) to least electronegative element (francium having electronegativity = 0.7) atomic number. elements.
Periodic Table With Electronegativity Values Labeled Image Electronegativity is the tendency of an atom to attract the electrons of another atom to form a bond. atoms with high electronegativity will strongly attract the electrons of other atoms. atoms with low electronegativity are more likely to have their bonding electrons pulled away. as you cross the periodic table from right to left. And this scale is known as pauling electronegativity scale.) the pauling electronegativity values of the element ranges from the most electronegative element (fluorine having electronegativity = 3.98) to least electronegative element (francium having electronegativity = 0.7) atomic number. elements.

Electronegativity Definition And Trend

Comments are closed.