Phase Change Transition Diagram States Matter Schema Evaporation
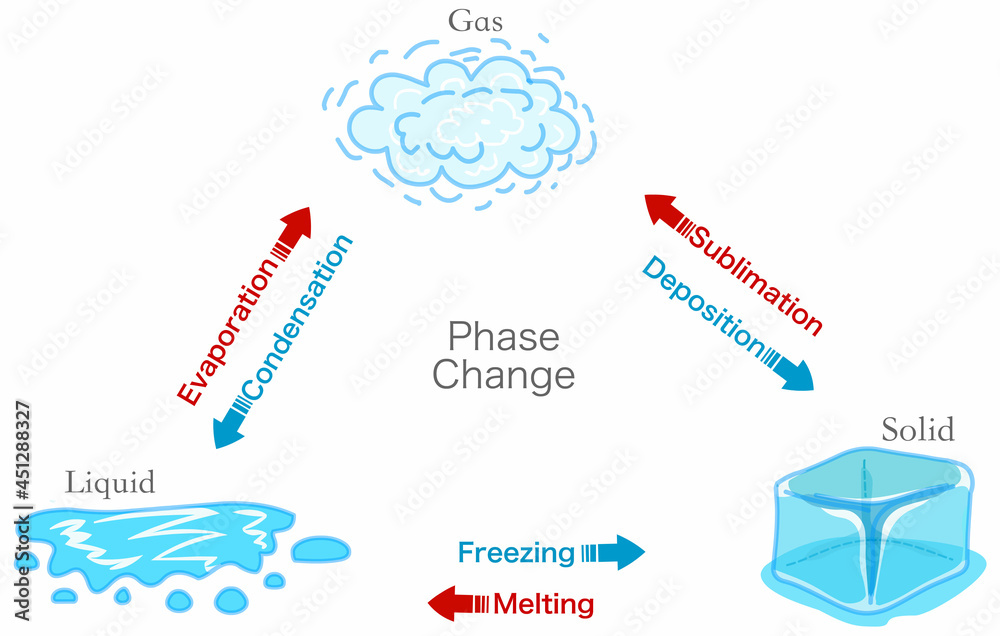
Phase Change Transition Diagram States Matter Schema Evaporation A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. the states of matter differ in the organization of particles and their energy. the main factors that cause phase changes are changes in temperature and pressure. at the phase transition, such as the boiling point between liquid. Phase changes. melting is defined as the process of converting a substance from the solid to the liquid state of matter. a chemical that exists in its liquid state can be changed back into a solid state in a process known as fusion, which is more commonly known as "freezing." melting and fusion are complementary phase changes, because both.

Phase Changes Of Matter Phase Transitions Such a pt graph is called a phase diagram. figure \(\pageindex{1}\) shows the phase diagram for water. using the graph, if you know the pressure and temperature, you can determine the phase of water. the solid curves—boundaries between phases—indicate phase transitions, that is, temperatures and pressures at which the phases coexist. Summary. phase transitions are processes that convert matter from one physical state into another. there are six phase transitions between the three phases of matter. melting, vaporization, and sublimation are all endothermic processes, requiring an input of heat to overcome intermolecular attractions. Sublimation. although the phase transition from solid to liquid (i.e., melting) and the one from liquid to gas (vaporizing) are the most commonly encountered ones, there are many other transitions that can occur. in particular, sublimation is when a substance undergoes a phase transition from a solid phase directly into a gaseous phase. There are six ways a substance can change between these three phases; melting, freezing, evaporating, condensing, sublimination, and deposition (2). these processes are reversible and each transfers between phases differently: melting: the transition from the solid to the liquid phase. freezing: the transition from the liquid phase to the solid.
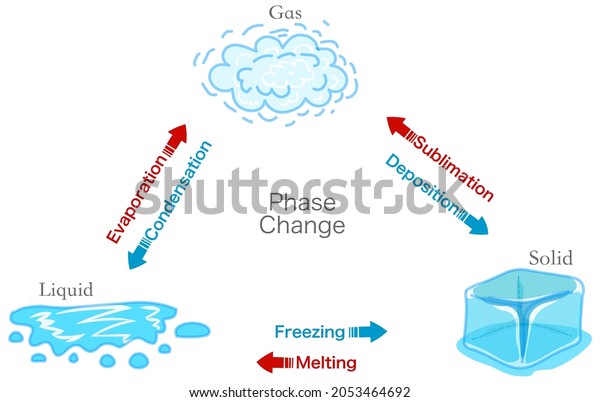
Phase Change Transition Diagram States Matter Stock Vector Royal Sublimation. although the phase transition from solid to liquid (i.e., melting) and the one from liquid to gas (vaporizing) are the most commonly encountered ones, there are many other transitions that can occur. in particular, sublimation is when a substance undergoes a phase transition from a solid phase directly into a gaseous phase. There are six ways a substance can change between these three phases; melting, freezing, evaporating, condensing, sublimination, and deposition (2). these processes are reversible and each transfers between phases differently: melting: the transition from the solid to the liquid phase. freezing: the transition from the liquid phase to the solid. Phase diagrams phases of matter and phase transitions. this is an example of a two dimensional phase diagram showing phase boundaries and colored coded phase regions. phases. at high pressures and low temperatures, the substance is in the solid phase. at low pressure and high temperature, the substance is in the gas phase. A phase diagram combines plots of pressure versus temperature for the liquid gas, solid liquid, and solid gas phase transition equilibria of a substance. these diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase transition temperatures.
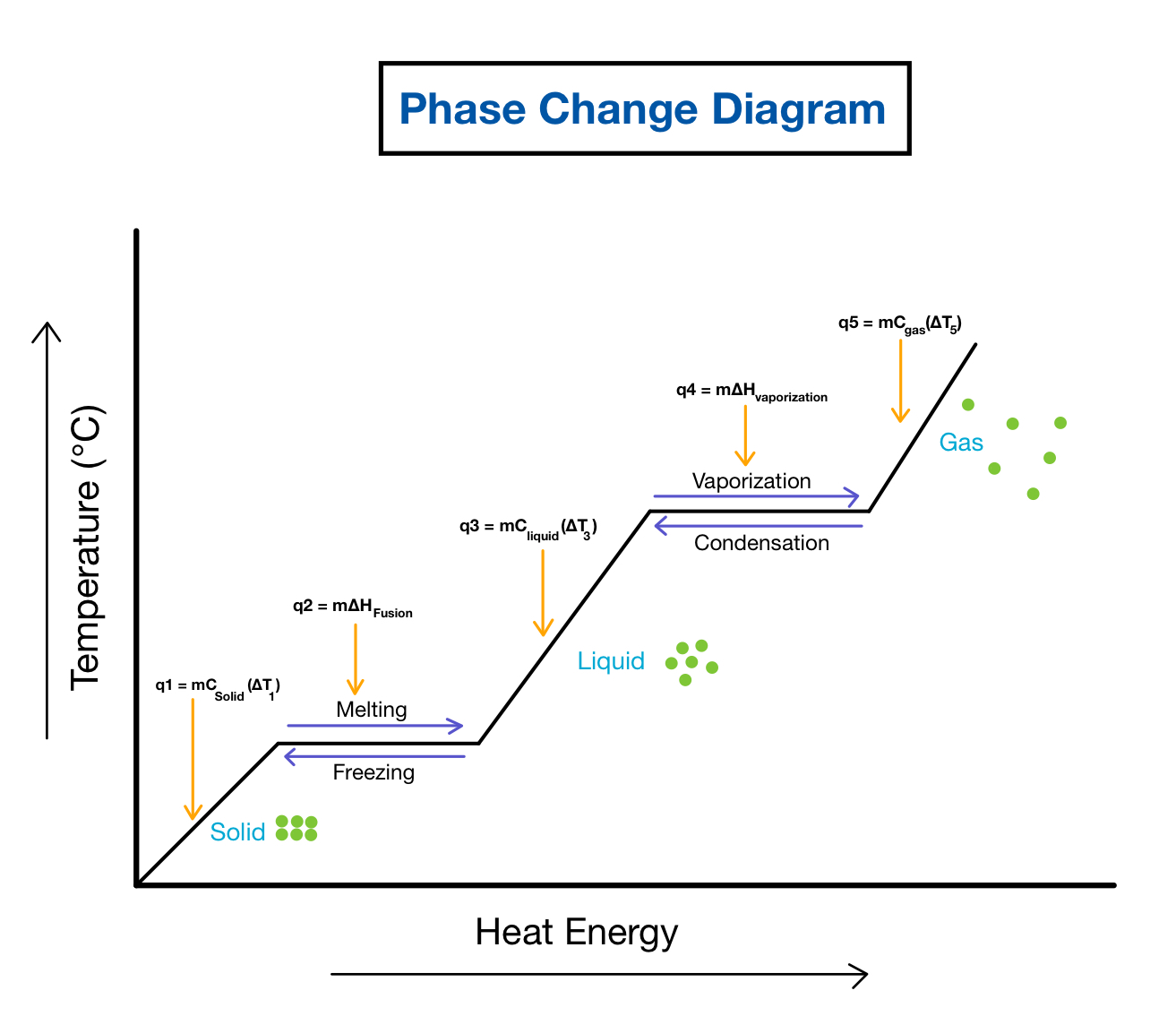
How To Interpret A Phase Change Diagram Phase diagrams phases of matter and phase transitions. this is an example of a two dimensional phase diagram showing phase boundaries and colored coded phase regions. phases. at high pressures and low temperatures, the substance is in the solid phase. at low pressure and high temperature, the substance is in the gas phase. A phase diagram combines plots of pressure versus temperature for the liquid gas, solid liquid, and solid gas phase transition equilibria of a substance. these diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase transition temperatures.
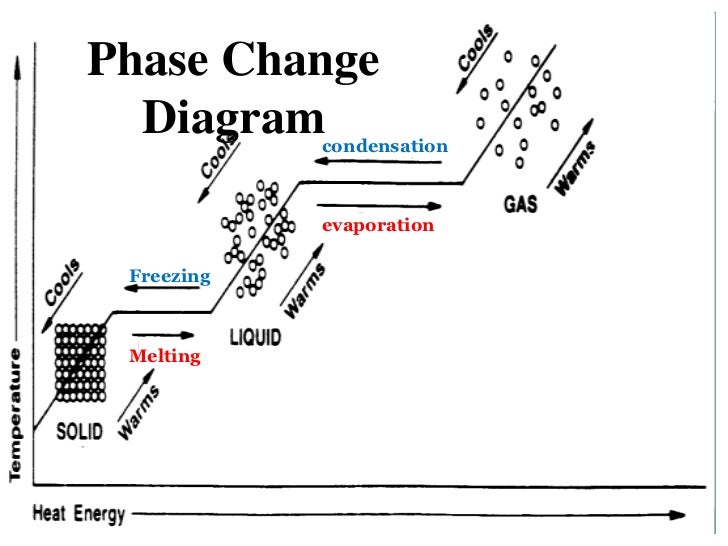
Phase Change Diagram Explained

Comments are closed.