Phase Diagrams Chemistry Libretexts

Phase Diagrams Chemistry Libretexts Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist. The phase diagram of water. figure 11.7.2 shows the phase diagram of water and illustrates that the triple point of water occurs at 0.01°c and 0.00604 atm (4.59 mmhg). far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point (273.16 k) is used to define the absolute (kelvin) temperature scale.

Phase Diagrams Chemistry Libretexts A generic phase diagram. the triple point and critical point are labeled. the solid green line represents the melting point of most liquids, and the dotted green line represents the unusual behavior of water. figure adapted from matthieumarechal via wikimedia commons. look at the diagram. Such a p t graph is called a phase diagram. [1] [1] in the phase diagram, we can observe that the boiling point curve (the line that distinguishes liquid and vapour phases) ends at a point, which is called critical point. above the critical point, liquid and gas can no longer be distinguished and are classified as a supercritical fluid. Figure chapter4.7: eutectic phase diagram. the binary eutectic phase diagram has several distinctive features one being a solid solid phase mixture, limit of solubility at different temperatures, and an invariant point in the phase diagram, the eutectic point. the solubility limit is the maximum amount of solute that you can integrate into the. Gibb’s phase rule is used to determine the number of intensive variables required to fully specify the thermodynamic properties of a system (thermodynamic degrees of freedom). once this number of intensive variables is specified, we can find all the thermodynamic properties of the substance (eg. other intensive variables such as pressure.
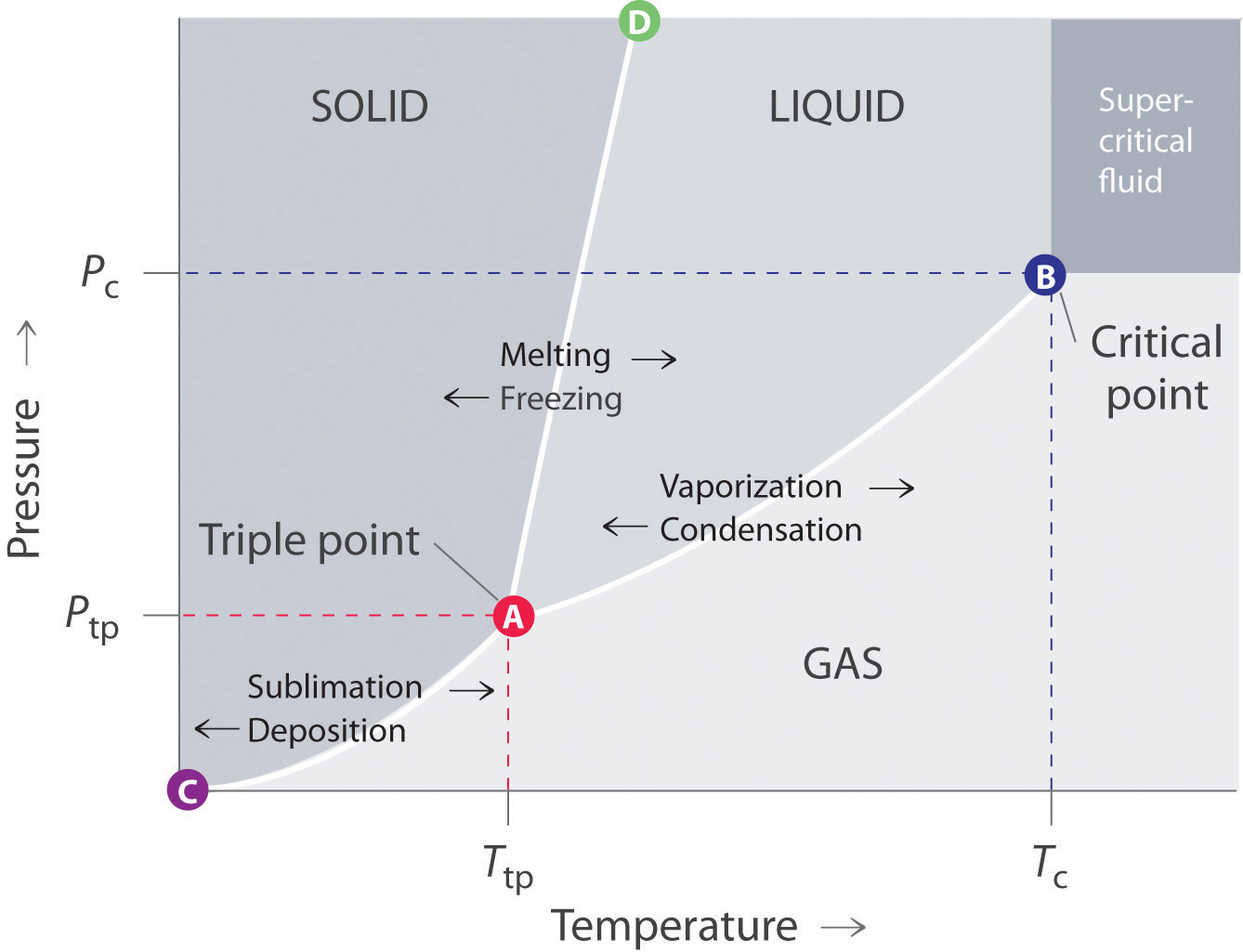
12 7 Phase Diagrams Chemistry Libretexts Figure chapter4.7: eutectic phase diagram. the binary eutectic phase diagram has several distinctive features one being a solid solid phase mixture, limit of solubility at different temperatures, and an invariant point in the phase diagram, the eutectic point. the solubility limit is the maximum amount of solute that you can integrate into the. Gibb’s phase rule is used to determine the number of intensive variables required to fully specify the thermodynamic properties of a system (thermodynamic degrees of freedom). once this number of intensive variables is specified, we can find all the thermodynamic properties of the substance (eg. other intensive variables such as pressure. The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. in figure 12.4.1 12.4. 1, the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with pressure. the solid and liquid phases are in. Phase diagram of water. critical pressure = p c = 22.06m p a p c = 22.06 m p a. critical temperature = t c = 647k t c = 647 k. triple point p p with ice i h i h = 611 pa. triple point t t with ice i h i h = 273.16 k. ice i h i h is the most common crystal structure of ice, but ice has a total of 17 known crystal forms.
.PNG)
Chapter 7 7 Phase Diagrams Chemistry Libretexts Vrogue Co The lines in a phase diagram correspond to the combinations of temperature and pressure at which two phases can coexist in equilibrium. in figure 12.4.1 12.4. 1, the line that connects points a and d separates the solid and liquid phases and shows how the melting point of a solid varies with pressure. the solid and liquid phases are in. Phase diagram of water. critical pressure = p c = 22.06m p a p c = 22.06 m p a. critical temperature = t c = 647k t c = 647 k. triple point p p with ice i h i h = 611 pa. triple point t t with ice i h i h = 273.16 k. ice i h i h is the most common crystal structure of ice, but ice has a total of 17 known crystal forms.

Comments are closed.