Phase Diagrams Introduction
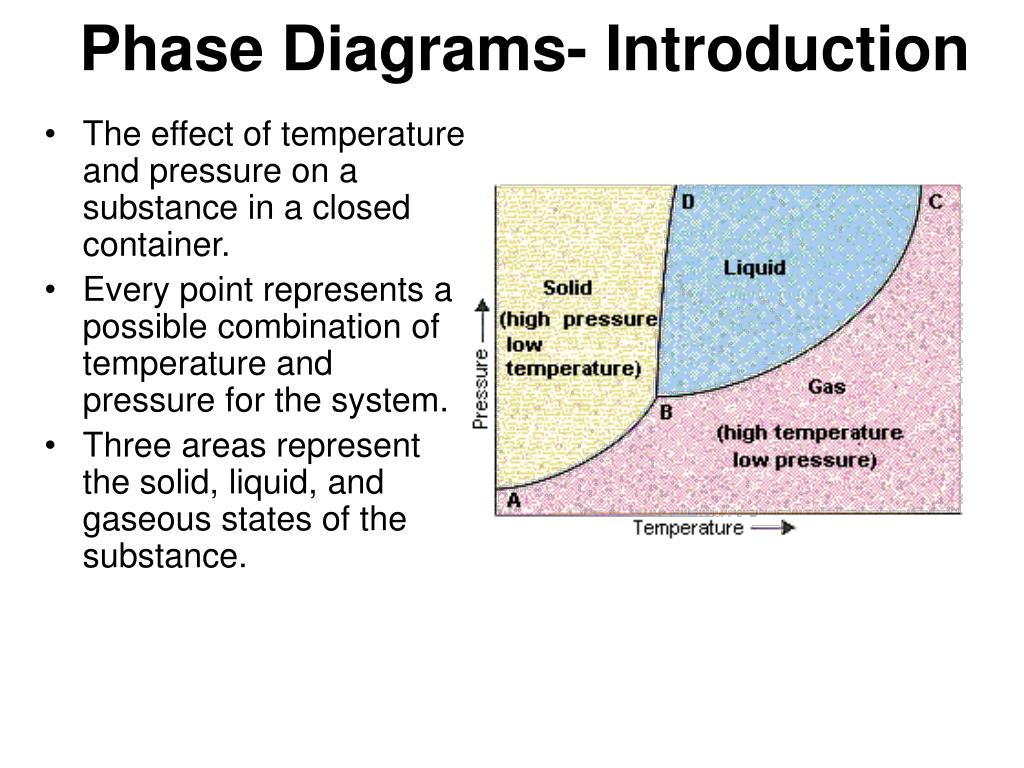
Introduction To Phase Diagrams A typical phase diagram for a pure substance is shown in figure 1. figure 1. the physical state of a substance and its phase transition temperatures are represented graphically in a phase diagram. to illustrate the utility of these plots, consider the phase diagram for water shown in figure 2. figure 2. Introduction. a phase diagram is a representation of different phases of a system consists of a substance or many substances at two different thermodynamic conditions such as temperature and pressure. phase diagram can also be drawn between other thermodynamic conditions such as between temperature and volume or temperature and solubility etc.

Phase Diagram Definition Explanation And Diagram Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. a typical phase diagram has pressure on the y axis and temperature on the x axis. as we cross the lines or curves on the phase diagram, a phase change occurs. in addition, two states of the substance coexist. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling. The phase diagram of carbon dioxide. in contrast to the phase diagram of water, the phase diagram of co 2 (figure 16.4.3 16.4. 3) has a more typical melting curve, sloping up and to the right. the triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. From the p t diagram, figure 2.3.e1, co 2 at 100 bar, 275 k is in the liquid phase. the isobaric process is shown as the horizontal, yellow line with a constant pressure of 100 bar, see figure 2.3.e1. at approximately 220 k, the isobaric process line meets the fusion line, and the liquid co 2 starts to change to solid co 2.
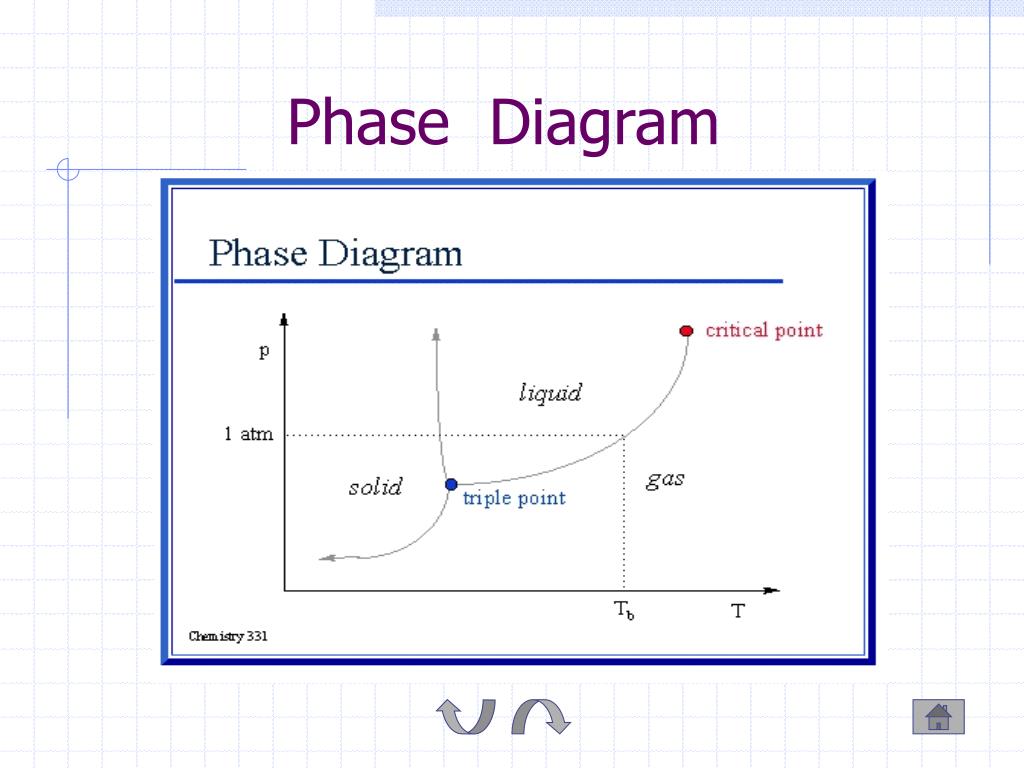
Diagram Phase Diagrams And Heterogeneous Equilibria A Practical The phase diagram of carbon dioxide. in contrast to the phase diagram of water, the phase diagram of co 2 (figure 16.4.3 16.4. 3) has a more typical melting curve, sloping up and to the right. the triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. From the p t diagram, figure 2.3.e1, co 2 at 100 bar, 275 k is in the liquid phase. the isobaric process is shown as the horizontal, yellow line with a constant pressure of 100 bar, see figure 2.3.e1. at approximately 220 k, the isobaric process line meets the fusion line, and the liquid co 2 starts to change to solid co 2. One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists. A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium.

Introduction To Phase Diagrams Youtube One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists. A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium.
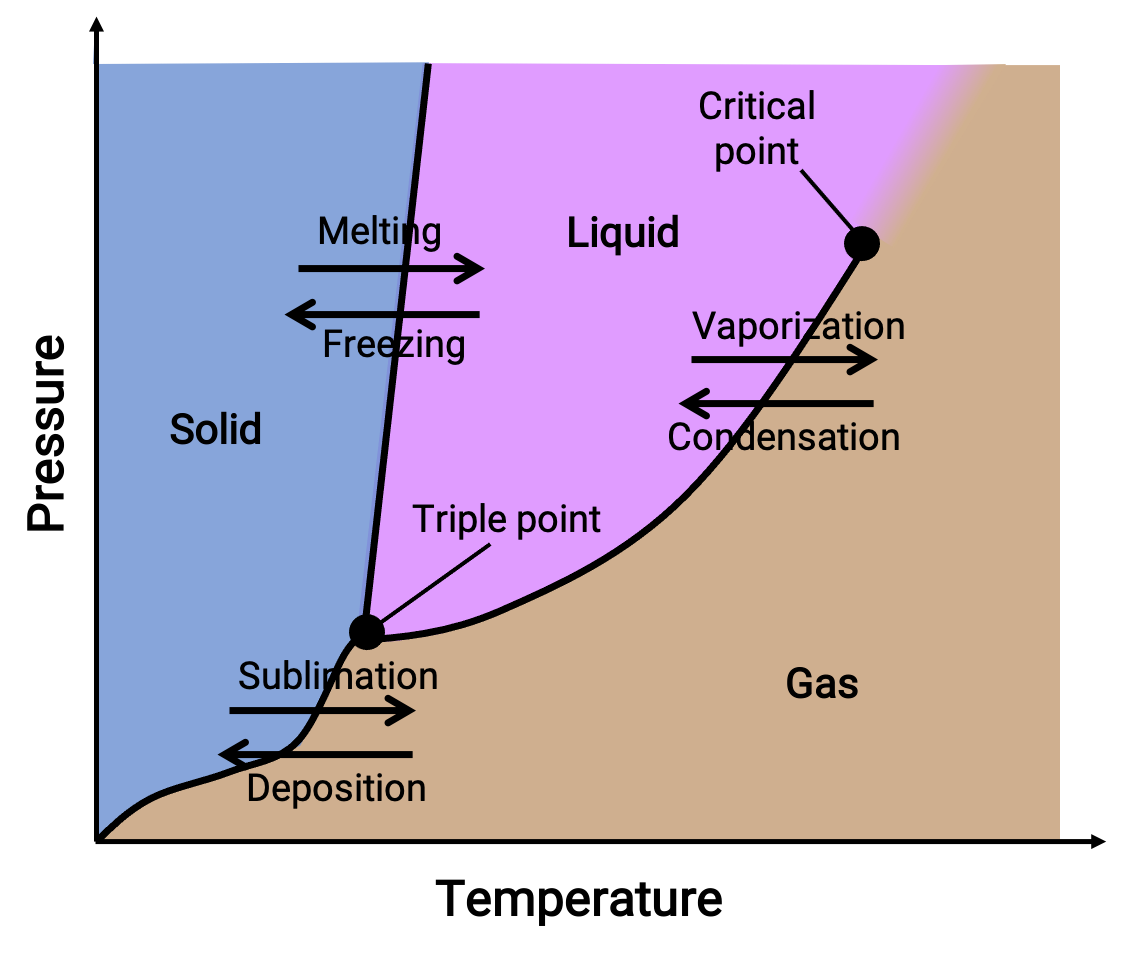
Phase Diagrams

Comments are closed.