Physicochemical Properties Of Drugs Oer Commons

Physicochemical Properties Of Drugs Oer Commons The illustration presents a mind map of the physicochemical properties of drugs which can be used by instructors to summarize the course. students can also use it to test their understanding of the subject matter. The illustration presents a mind map of the physicochemical properties of drugs which can be used by instructors to summarize the course. students can also use it to test their understanding of the subject matter. subject: health, medicine and nursing level: graduate professional material type: teaching learning strategy author: gladys mokua.
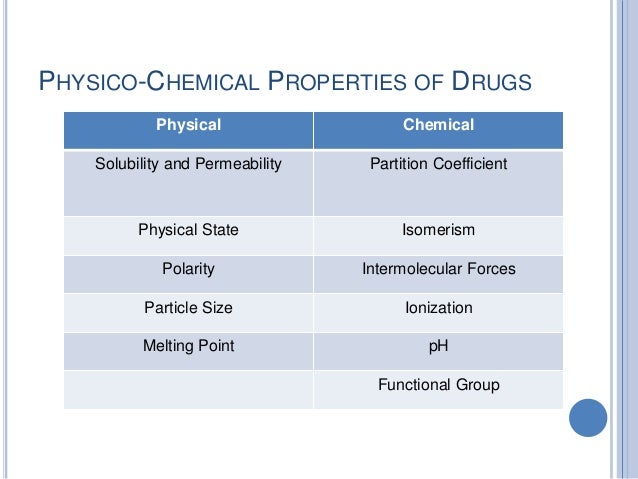
1 Lab Physico Chemical Properties Of Drugs 1 Atinnin. study with quizlet and memorize flashcards containing terms like most drugs in medicine behave like strong or weak acids and bases?, can you determine if the compound is an acid or base based on the pka or pkb?, what information do you get from the pka or pkb? and more. Lecture 1: physicochemical properties of drugs and drug disposition key objectives: 1. be able to explain the benefits of oral versus iv drug administration 2. be able to explain the factors involved in drug absorption 3. be able to state if a drug possesses a charge at a given ph value 4. The guidance for three key properties for development of oral drugs which emerged from this study is dose ≤100 mg, fassif solubility ≥100 µg ml −1 and pfi ≤6. 81 fassif solubility is a biorelevant solubility measure in fasted state simulated intestinal fluid. 36 as an indication that the control of physicochemical properties can. Besides lipophilicity, physicochemical properties of importance for binding include molecular size, hydrogen bond acceptors donors and charge. this chapter discusses the physicochemical properties of importance for drug metabolism. the primary organ for drug metabolism is the liver and to reach the liver the compound must cross cellular barriers.

Physicochemical Properties Of Drugs Download Table The guidance for three key properties for development of oral drugs which emerged from this study is dose ≤100 mg, fassif solubility ≥100 µg ml −1 and pfi ≤6. 81 fassif solubility is a biorelevant solubility measure in fasted state simulated intestinal fluid. 36 as an indication that the control of physicochemical properties can. Besides lipophilicity, physicochemical properties of importance for binding include molecular size, hydrogen bond acceptors donors and charge. this chapter discusses the physicochemical properties of importance for drug metabolism. the primary organ for drug metabolism is the liver and to reach the liver the compound must cross cellular barriers. Physicochemical properties of drugs 61 applying knowledge of the pka and an appreciation of the extent to which drugs ionise in solution. deductions of this type form the basis ofmedicinal chemistry, the science of rational drug design. phenols another commonly encountered acidic functional group found in drug molecules is phenol, or. Abstract. this chapter provides a description of the three central elements in the physicochemical characterization of drug compounds, i.e., the acid dissociation constant, log p, and solubility, and the associated experiment to determine the solid form, in particular, for the latter property. the description of the different properties.

Molecular Properties Of Drugs Pharmaceutical Medicinal Chemistry I Physicochemical properties of drugs 61 applying knowledge of the pka and an appreciation of the extent to which drugs ionise in solution. deductions of this type form the basis ofmedicinal chemistry, the science of rational drug design. phenols another commonly encountered acidic functional group found in drug molecules is phenol, or. Abstract. this chapter provides a description of the three central elements in the physicochemical characterization of drug compounds, i.e., the acid dissociation constant, log p, and solubility, and the associated experiment to determine the solid form, in particular, for the latter property. the description of the different properties.

Physicochemical Properties Of Drug Youtube

Comments are closed.