Principles Of Documenting Data
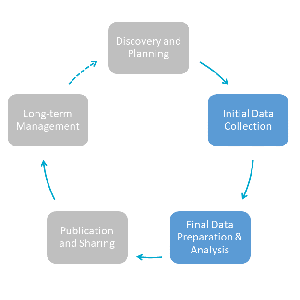
Principles Of Documenting Data At the core of good data management is documentation. documentation introduces your data, provides a detailed description of their key attributes, and contextualizes them your documentation should describe what you did and why you made the choices you made. documentation can be written at many “levels” and comes in many forms (as described. Data documentation serves several crucial purposes in the context of information management, data analysis, and decision making. here are some key reasons why documenting data is important: 1. enhanced understanding. data documentation provides a clear and detailed understanding of the data, fostering transparency and reducing ambiguity.
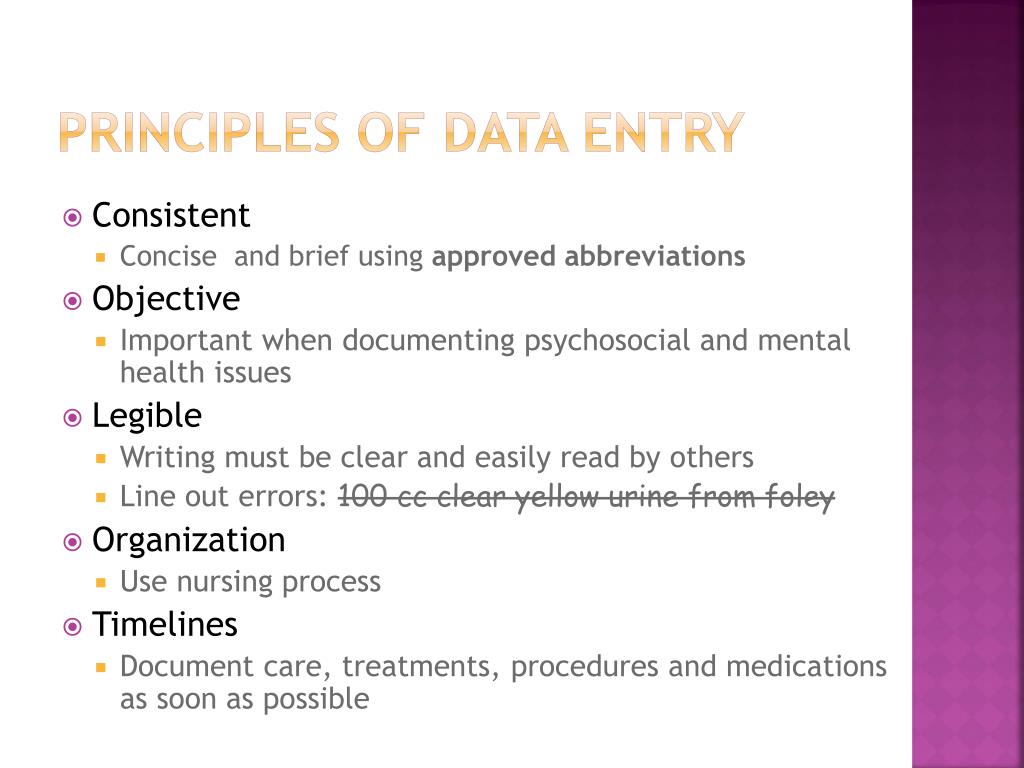
Ppt Documenting Reporting Nursing Informatics Powerpoint Presentation What matters is that all researchers manage their data and documentation in ways that meet the increasingly strict standards that funding agencies, journals, and the field of scientific psychology at large are adopting to promote greater transparency and replicability of psychological research. Documenting methods and describing data. this page is designed to help you: document protocols and methods used to collect and analyze data to enable replication of your methods. provide contextual information for data to aid discovery, access, citation, and reuse of your data. use international standards for uniform description of data. Roots of good documentation principles are in the ich gcp where source data and source document is first defined. ich e6 1.51 source data all information in original records and certified copies of original records of clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the. Good documentation practices (gdocp) consists of a set of guidelines for creating, maintaining, and managing documents. while generally associated with the research and development of pharmaceuticals and medical devices, this set of standards isn’t confined to a specific industry. organizations in any business or government sector — it.

Chapter 4 Validating And Documenting Data Chapter 4 Validating And Roots of good documentation principles are in the ich gcp where source data and source document is first defined. ich e6 1.51 source data all information in original records and certified copies of original records of clinical findings, observations, or other activities in a clinical trial necessary for the reconstruction and evaluation of the. Good documentation practices (gdocp) consists of a set of guidelines for creating, maintaining, and managing documents. while generally associated with the research and development of pharmaceuticals and medical devices, this set of standards isn’t confined to a specific industry. organizations in any business or government sector — it. When documenting unstructured data elements, the same considerations as paper records apply. table 4 focuses on accountability: the requirement that “nurses are accountable for ensuring their documentation of client care is accurate, timely and complete” (cno, 2019a, p. 7). Ana’s principles for nursing documentation identifies six essential principles to guide nurses in this necessary and integral aspect of the work of registered nurses in all roles and settings. american nurses association 8515 georgia avenue, suite 400 silver spring, md 20910 3492. 1 800 274 4ana.

Comments are closed.