Randomized Controlled Trials вђ Howmed
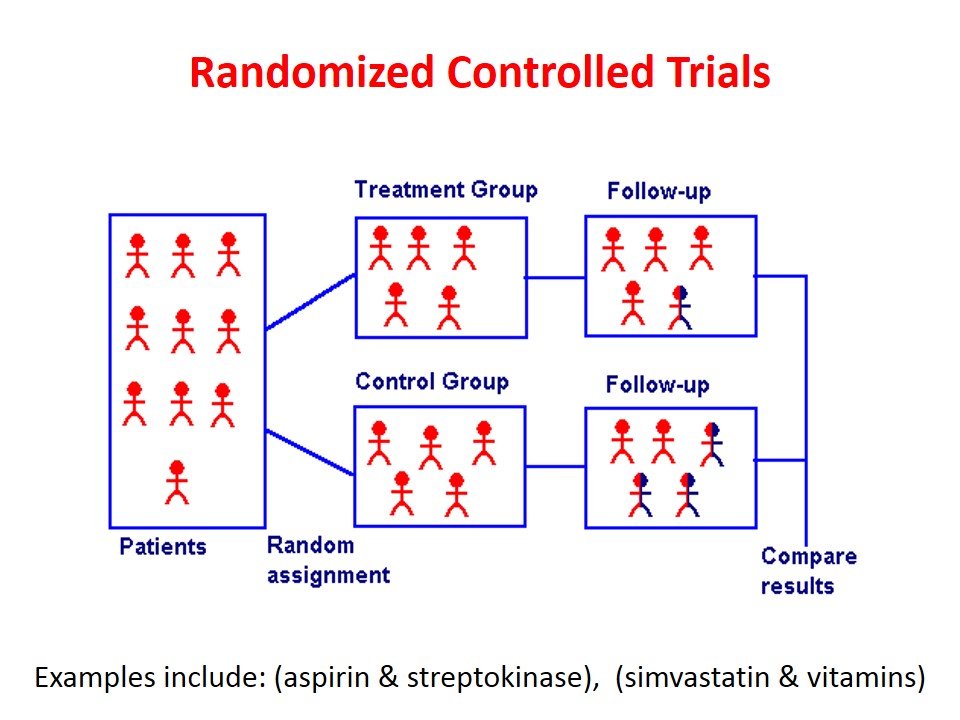
Randomized Controlled Trials вђ Howmed Randomized controlled trials (rcts) have traditionally been viewed as the gold standard of clinical trial design, residing at the top of the hierarchy of levels of evidence in clinical study; this is because the process of randomization can minimize differences in characteristics of the groups that may influence the outcome, thus providing the. The strengths of rcts are subtle: they are powerful because of the set of procedures that they are expected to follow. this includes the use of controls, placebos, experimentation, randomization, concealment, blinding, intention to treat analysis, and pre registration.
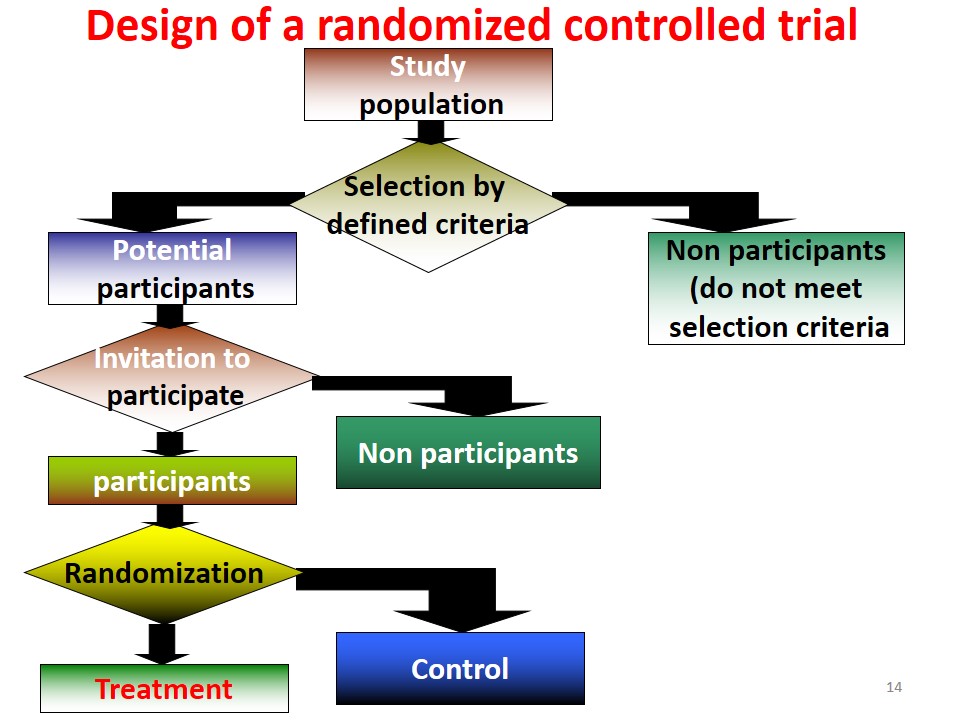
Randomized Controlled Trials вђ Howmed Randomized controlled trials (rcts) have traditionally been considered the gold standard for medical evidence. however, in light of emerging methodologies in data science, many experts question the role of rcts. within this context, experts in the usa and canada came together to debate whether the primacy of rcts as the gold standard for medical evidence, still holds in light of recent. A randomized controlled trial (rct) is a prospective experimental design that randomly assigns participants to an experimental or control group. rcts are the gold standard for establishing causal relationships and ruling out confounding variables and selection bias. researchers must be able to control who receives the treatments and who are the. This chapter describes methodological and design considerations central to the scientific evaluation of clinical treatment methods via randomized clinical trials (rcts). matters of design, procedure, measurement, data analysis, and reporting are each considered in turn. Randomized controlled trials (rcts) are considered the highest level of evidence to establish causal associations in clinical research. there are many rct designs and features that can be selected to address a research hypothesis. designs of rcts have become increasingly diverse as new methods have been proposed to evaluate increasingly complex.

Randomized Control Trial Rct Simply Psychology This chapter describes methodological and design considerations central to the scientific evaluation of clinical treatment methods via randomized clinical trials (rcts). matters of design, procedure, measurement, data analysis, and reporting are each considered in turn. Randomized controlled trials (rcts) are considered the highest level of evidence to establish causal associations in clinical research. there are many rct designs and features that can be selected to address a research hypothesis. designs of rcts have become increasingly diverse as new methods have been proposed to evaluate increasingly complex. Randomised controlled trials (rcts) are the workhorse of evidence based healthcare and the only research design that can demonstrate causality, that is, that an intervention causes a. Abstract. randomized controlled trials (rcts) are the hallmark of evidence based medicine and form the basis for translating research data into clinical practice. this review summarizes commonly applied designs and quality indicators of rcts to provide guidance in interpreting and critically evaluating clinical research data.

Illustration Of A Randomised Controlled Trial Rct To Test A New к Back Randomised controlled trials (rcts) are the workhorse of evidence based healthcare and the only research design that can demonstrate causality, that is, that an intervention causes a. Abstract. randomized controlled trials (rcts) are the hallmark of evidence based medicine and form the basis for translating research data into clinical practice. this review summarizes commonly applied designs and quality indicators of rcts to provide guidance in interpreting and critically evaluating clinical research data.

Comments are closed.