Read What Is The Stoichiometry Of Hc2h3o2 To Ca Oh 2 Updated 2021
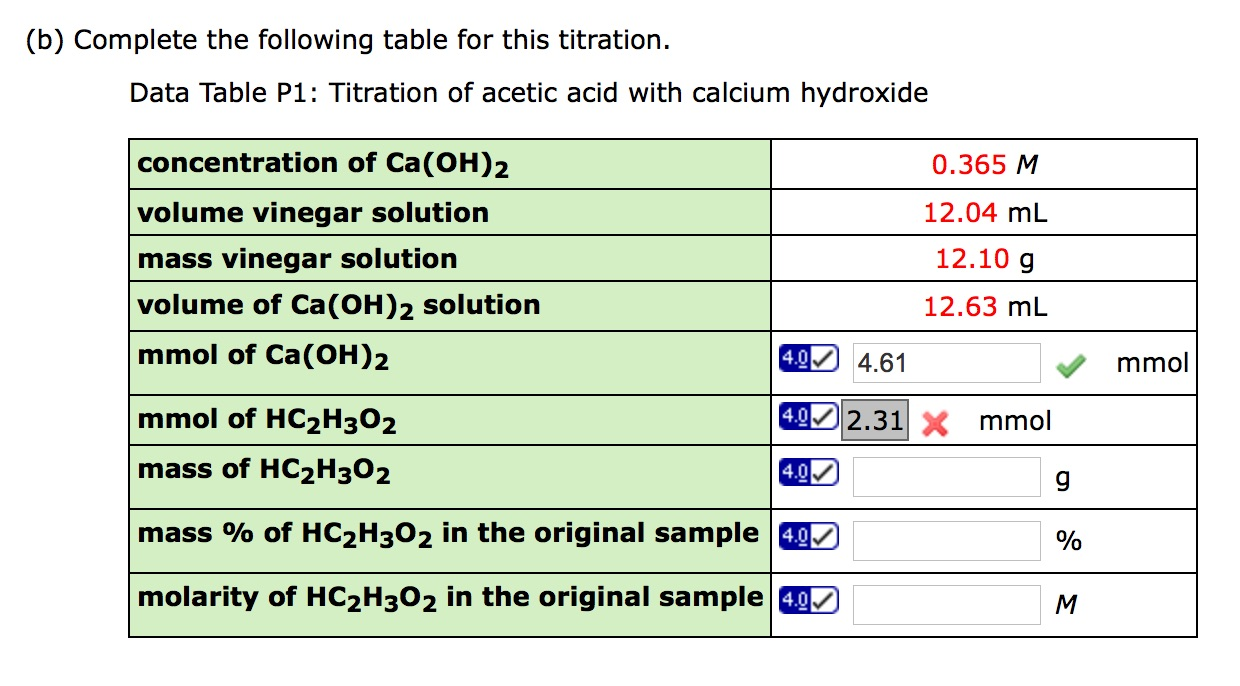
Read What Is The Stoichiometry Of Hc2h3o2 To Ca Oh 2 Updated 2021 (a) what is the stoichiometry of hc2h3o2 to ca(oh)2 ? \begin{tabular}{|c|} \hline 8:3 \\ \hline 5:2 \\ \hline 2:1 \\ 1:1 \\ \hline 1:2 \\ 2:5 \\ 3:8 \\ \hline \end{tabular} (b) complete the following table for this titration. data table p1: titration of acetic acid with calcium hydroxide. The stoichiometry of hc2h3o2 to ca(oh)2 is 2:1. explanation: the stoichiometry of hc2h3o2 to ca(oh)2 can be determined by looking at the balanced chemical equation: 2 hno3 ca(oh)2 → ca(no3)2 2 h2o. from the equation, we can see that for every 1 mol of ca(oh)2, we need 2 mol of hc2h3o2. therefore, the stoichiometry of hc2h3o2 to ca(oh)2 is.

Read What Is The Stoichiometry Of Hc2h3o2 To Ca Oh 2 Updated 2021 2,184 solutions. find step by step chemistry solutions and your answer to the following textbook question: consider a different titrant for this exercise. suppose ca (oh)2 were used as the titrant, instead of naoh. this will make the titrant twice as concentrated in hydroxide ion. the analyte will still be hc2h3o2. Calcium (ca) has an oxidation number of 0 in its elemental form. phosphorus (p) also has an oxidation number of 0 in its elemental form. in ca 3 p 2, calcium has an oxidation number of 2, and phosphorus has an oxidation number of 3. identify the changes in oxidation numbers: calcium goes from 0 to 2, losing 2 electrons (reduction). How many nh 3 molecules are produced by the reaction of 4.0 mol of ca (oh) 2 according to the following equation: (nh 4) 2 so 4 ca (oh) 2 2 nh 3 caso 4 2 h 2 o. answer: 4.8 × 10 24 nh 3 molecules. these examples illustrate the ease with which the amounts of substances involved in a chemical reaction of known stoichiometry may be related. Textbook question. complete and balance each acid–base equation. b. hc2h3o2 (aq) ca (oh)2 (aq)¡. verified solution. this video solution was recommended by our tutors as helpful for the problem above. 3m. 986. mark as completed.

A What Is The Stoichiometry Of Hc2h3o2 To Ca Oh 2 Chegg How many nh 3 molecules are produced by the reaction of 4.0 mol of ca (oh) 2 according to the following equation: (nh 4) 2 so 4 ca (oh) 2 2 nh 3 caso 4 2 h 2 o. answer: 4.8 × 10 24 nh 3 molecules. these examples illustrate the ease with which the amounts of substances involved in a chemical reaction of known stoichiometry may be related. Textbook question. complete and balance each acid–base equation. b. hc2h3o2 (aq) ca (oh)2 (aq)¡. verified solution. this video solution was recommended by our tutors as helpful for the problem above. 3m. 986. mark as completed. What is the stoichiometry of hc2h3o2 to ca(oh)2? 1:1 1.2 2.5 0.38 (b) complete the following table for this titration: data table: titration of acetic acid with calcium hydroxide concentration (ca(oh)2) volume of vinegar solution (ml) volume of ca(oh)2 solution (ml) mmol of ca(oh)2 mmol of hc2h3o2 % of hc2h3o2 in the original sample molarity of. Write the molecular, total ionic, and net ionic equations for the following reactions:ca(oh)2(aq) hc2h3o2(aq) →openstax™ is a registered trademark, which w.

Solved A What Is The Stoichiometry Of Hc2h3o2 To Ca Oh 2 Chegg What is the stoichiometry of hc2h3o2 to ca(oh)2? 1:1 1.2 2.5 0.38 (b) complete the following table for this titration: data table: titration of acetic acid with calcium hydroxide concentration (ca(oh)2) volume of vinegar solution (ml) volume of ca(oh)2 solution (ml) mmol of ca(oh)2 mmol of hc2h3o2 % of hc2h3o2 in the original sample molarity of. Write the molecular, total ionic, and net ionic equations for the following reactions:ca(oh)2(aq) hc2h3o2(aq) →openstax™ is a registered trademark, which w.

Comments are closed.