Regulatory Affairs Introduction To Ctd

Regulatory Affairs Introduction To Ctd Pdf Document Regulatory authorities. modules 2–5 though are common to all regions and these comprise the main body of the ctd. module 2 contains the ctd overviews and sum maries. it starts with a general introduction to the drug, including its pharmacological class, mode of action, and proposed clinical use. module 2 then. The ctd format is required by regulatory authorities, such as the fda in the us and the ema in the eu, for the registration and approval of new drugs and for post approval regulatory submissions.
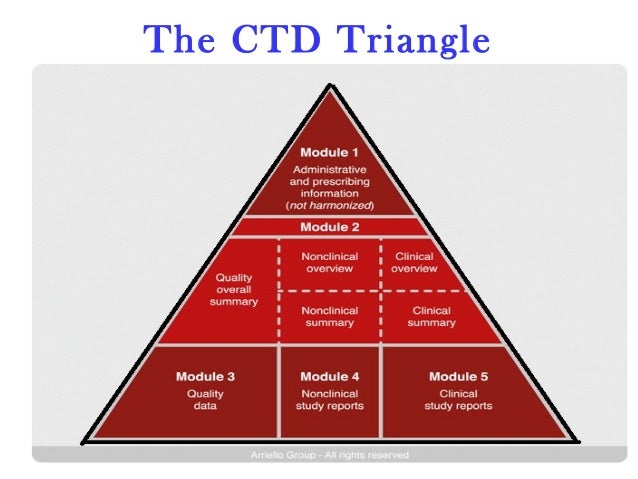
Regulatory Affairs Introduction To Ctd Module 2 contains the ctd summaries and should begin with a general introduction to the drug, including its pharmacological class, mode of action and proposed clinical use. module 2 should also provide the overall summary of the ‘quality’ information provided, the non clinical overview and the clinical overview, as well as the non clinical written summaries and the tabulated summaries, and. Ich guideline m4 (r4) on common technical document (ctd) for the registration of pharmaceuticals for human use organisation of ctd step 5 adopted reference number: cpmp ich 2887 99 english (en) (438.43 kb pdf). The common technical document (ctd) format is a standard format for submitting regulatory information on pharmaceuticals to regulatory authorities, such as the u.s. food and drug administration (fda) and the european medicines agency (ema). here are some frequently asked questions to help you understand the ctd format in pharmaceutical. Module 2: common technical document (ctd) summaries (see ich guidelines m4q, m4s, m4e) 2.1 common technical document table of contents (modules 2‒5) 2.2 ctd introduction. 2.3 quality overall summary. 2.4 nonclinical overview. 2.5 clinical overview. 2.6 nonclinical written and tabulated summaries: pharmacology, pharmacokinetics, toxicology.

Regulatory Affairs Introduction To Ctd The common technical document (ctd) format is a standard format for submitting regulatory information on pharmaceuticals to regulatory authorities, such as the u.s. food and drug administration (fda) and the european medicines agency (ema). here are some frequently asked questions to help you understand the ctd format in pharmaceutical. Module 2: common technical document (ctd) summaries (see ich guidelines m4q, m4s, m4e) 2.1 common technical document table of contents (modules 2‒5) 2.2 ctd introduction. 2.3 quality overall summary. 2.4 nonclinical overview. 2.5 clinical overview. 2.6 nonclinical written and tabulated summaries: pharmacology, pharmacokinetics, toxicology. We interviewed our regulatory affairs trainer, sophie nageotte, to discuss the ctd. she explains what it is in simple terms and provides some insight into the most challenging areas such as module 3 of the ctd (cmc quality module). could you give us a quick summary of the ich ctd (common technical document. what is it in regulatory affairs?. The ctd is an internationally agreed format for the preparation of applications regarding new drugs intended to be submitted to regional regulatory authorities in participating countries. it was developed by the european medicines agency (ema, europe), the food and drug administration and the ministry of health, labour and welfare (japan.

Comments are closed.