Session 41 Metallurgy Equilibrium Cooling Of Various Types Of

Session 41 Metallurgy Equilibrium Cooling Of Various Types Of Session 41 metallurgy equilibrium cooling of various types of steels free download as pdf file (.pdf), text file (.txt) or read online for free. In this article we will discuss about: 1. introduction to the iron carbon equilibrium diagram 2. phases in fe fe 3 c diagram 3. critical temperatures 4.transformations and microstructures of slowly cooled steels 5. methods used to distinguish between free ferrite and free cementite 6.

Practical Maintenance Blog Archive Phase Diagrams Part 1 A system with f = 2 is bivariant. therefore, the system may be in equilibrium at different temperatures and concentrations. it may be noted that: 1. the phase rule applies to dynamic and reversible processes, where a system is heterogeneous and in equilibrium and where the only external variables are pressure, temperature and concentration. 2. An equilibrium phase diagram is a diagram that shows the natural tendencies of a material or material system. pressure, temperature, and composition are important. transitions are encountered when a material changes phase. sublimation occurs when a material goes from a solid to a gas. freeze drying operates on this principle. Formed by extensive heat treatment around 900 deg c, fe3c will dissociate and form irregular shaped graphite nodules. rapid cooling restricts production amount to up to 5 kg. less voids and notches. ferritic mci: 10% el,35 ksi yield strength, 50 ksi tensile strength. There are a number of different types of thermal equilibrium diagrams 1. an alloy system in which the two metals are soluble in each other in all proportions in both liquid and solid state. 2. an alloy system in which the two metals are soluble in each other in all proportions in the liquid but not in the solid state. 3.
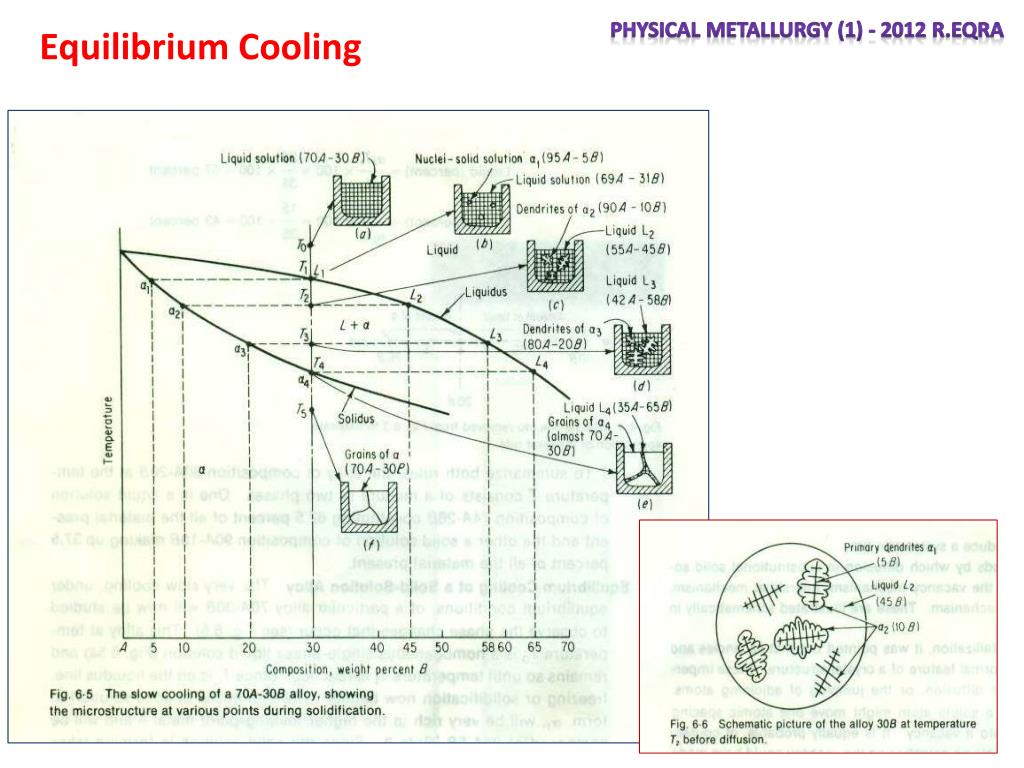
Ppt Phases And The Phase Diagram Powerpoint Presentation Free Formed by extensive heat treatment around 900 deg c, fe3c will dissociate and form irregular shaped graphite nodules. rapid cooling restricts production amount to up to 5 kg. less voids and notches. ferritic mci: 10% el,35 ksi yield strength, 50 ksi tensile strength. There are a number of different types of thermal equilibrium diagrams 1. an alloy system in which the two metals are soluble in each other in all proportions in both liquid and solid state. 2. an alloy system in which the two metals are soluble in each other in all proportions in the liquid but not in the solid state. 3. Transformation of austenite or continuous cooling at various rate. this diagram is called continuous cooling diagram. 5. continuous cooling shifts the beginning of austenite transformation of lower temperature and for longer time. 6. so, must of industrial heat treatment of steels are carried out by continuous cooling. 12.5: interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point.

Metallurgy Of Carbon Steel Transformation of austenite or continuous cooling at various rate. this diagram is called continuous cooling diagram. 5. continuous cooling shifts the beginning of austenite transformation of lower temperature and for longer time. 6. so, must of industrial heat treatment of steels are carried out by continuous cooling. 12.5: interpretation of cooling curves. the melting temperature of any pure material (a one component system) at constant pressure is a single unique temperature. the liquid and solid phases exist together in equilibrium only at this temperature. when cooled, the temperature of the molten material will steadily decrease until the melting point.

Thermal Equilibrium Diagram Material And Metallurgy Hsbte Youtube

Comments are closed.