Solute And Solvent Difference Difference Between Solute And S
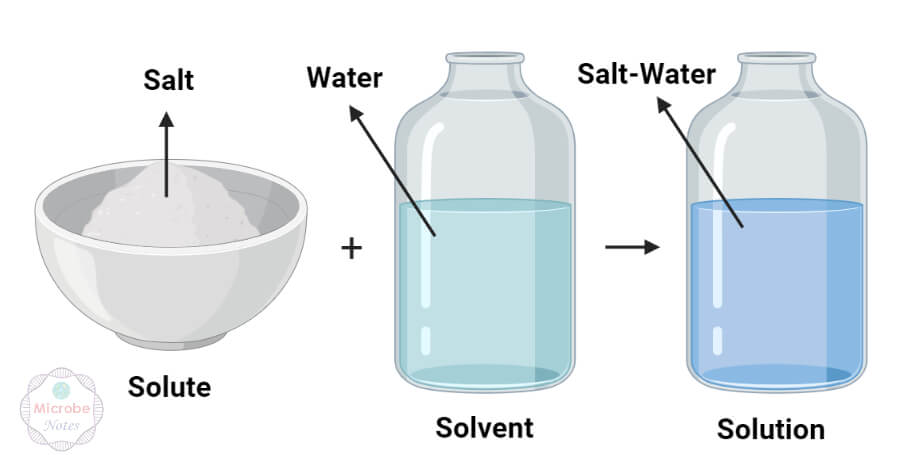
Solute Vs Solvent Definition 9 Major Differences Examples A solute can take many forms. it may be in the form of a gas, a liquid, or a solid. the part of a solution that is present in the greatest amount is called a solvent. it’s the liquid that the solute is dissolved in. a solvent is usually a liquid. phase. the dispersed step of a solution is known as the solute. The solute and solvent are the two prime components of the solution. the solute is one that gets dissolved. while the solvent is one in which the former dissolves. in other words, the solute is dissolved matter, and the solvent is the dissolving medium. the solutes are present in all three states, i.e., solid, liquid and gas.

Solute Vs Solvent Biology Lessons Solvent Science Education The main difference between solute and solvent is that a solute is a substance that is added to a solvent to form a solution. a solvent is a substance that dissolves the solute particles during the formation of a solution. let us now understand more about the difference between solute and solvent by studying in detail. Solute and solvent are two essential components of a solution. the solute refers to the substance that is dissolved in a solution, while the solvent is the substance that dissolves the solute. the solute is typically present in a smaller quantity compared to the solvent. it can be a solid, liquid, or gas, depending on the nature of the solution. Figure 15.4.1 15.4. 1: typical solution: clear and stable. in order to be a true solution, a mixture must be stable. when sugar is fully dissolved into water, it can stand for an indefinite amount of time and the sugar will not settle out of the solution. further, if the sugar water solution is passed through a filter, it will be unchanged. The solutions that are exemplified in exercise 7.2.1 7.2. 1 contain solid, liquid, and gaseous solutes and solvents. as shown below in table 7.2.1 7.2. 1, solutions can be prepared using solvents and solutes in any state of matter combination, and the physical form of the resultant solution corresponds to the phase of its constituent solvent.

Solute And Solvent Difference Difference Between Solute And S Figure 15.4.1 15.4. 1: typical solution: clear and stable. in order to be a true solution, a mixture must be stable. when sugar is fully dissolved into water, it can stand for an indefinite amount of time and the sugar will not settle out of the solution. further, if the sugar water solution is passed through a filter, it will be unchanged. The solutions that are exemplified in exercise 7.2.1 7.2. 1 contain solid, liquid, and gaseous solutes and solvents. as shown below in table 7.2.1 7.2. 1, solutions can be prepared using solvents and solutes in any state of matter combination, and the physical form of the resultant solution corresponds to the phase of its constituent solvent. Solvents that are nonpolar will dissolve nonpolar solutes. thus water, being polar, is a good solvent for ionic compounds and polar solutes like ethanol (c 2 h 5 oh). however, water does not dissolve nonpolar solutes, such as many oils and greases (figure 11.1.1 11.1. 1). figure 11.1.1 11.1. 1: a beaker holds water with blue food dye (upper. Next post →. what is the difference between a solvent and a solute? both solvent and solute are parts of a solution. solutions are mixtures of two or more substances, and the substance that dissolves into the solution is a solute. meanwhile, the solute dissolves into a substance called the solvent. solutes and solvents are mixed together to.

Difference Between Solute And Solvent Youtube Solvents that are nonpolar will dissolve nonpolar solutes. thus water, being polar, is a good solvent for ionic compounds and polar solutes like ethanol (c 2 h 5 oh). however, water does not dissolve nonpolar solutes, such as many oils and greases (figure 11.1.1 11.1. 1). figure 11.1.1 11.1. 1: a beaker holds water with blue food dye (upper. Next post →. what is the difference between a solvent and a solute? both solvent and solute are parts of a solution. solutions are mixtures of two or more substances, and the substance that dissolves into the solution is a solute. meanwhile, the solute dissolves into a substance called the solvent. solutes and solvents are mixed together to.

Solute Vs Solvent Definition 10 Major Differences And Examples The

Comments are closed.