Solute Vs Solvent Definition Difference Between Solute And
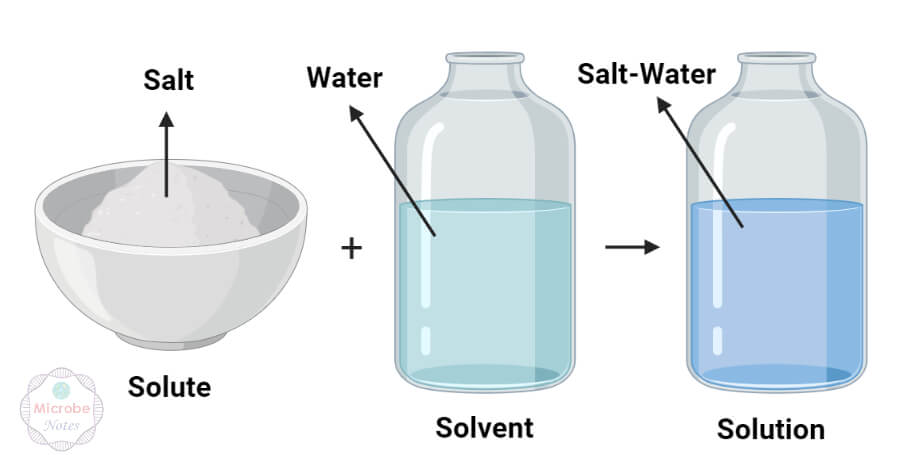
Solute Vs Solvent Definition 9 Major Differences Examples A solute can take many forms. it may be in the form of a gas, a liquid, or a solid. the part of a solution that is present in the greatest amount is called a solvent. it’s the liquid that the solute is dissolved in. a solvent is usually a liquid. phase. the dispersed step of a solution is known as the solute. Definition. a solute is a substance that dissolves with solvent to form a solution. the solvent is a substance in which solute dissolves during the formation of the solution. the solvent is usually a liquid. phase. a solute is the dispersed phase of the solution. the solvent is the solution’s medium phase, which disperses the solute particles.

Difference Between Solvent And Solute Definition Properties Examples Solvents that are nonpolar will dissolve nonpolar solutes. thus water, being polar, is a good solvent for ionic compounds and polar solutes like ethanol (c 2 h 5 oh). however, water does not dissolve nonpolar solutes, such as many oils and greases (figure 11.1.1 11.1. 1). figure 11.1.1 11.1. 1: a beaker holds water with blue food dye (upper. A solution is a homogeneous mixture consisting of a solute dissolved into a solvent. the solute is the substance that is being dissolved, while the solvent is the dissolving medium. solutions can be formed with many different types and forms of solutes and solvents. we know of many types of solutions. check out a few examples in the table below. A solute is a molecule or particle that is distributed in a solvent. there will always be less solute than solvent. that means in a solution the solute is always the minor component. typically, the solute will be uniformly distributed in the solvent after mixing. practically, the solute is also usually being added to the solvent. The solute is the dispersed phase of a solution. the solvent is the medium phase of a solution that disperses solute particles. the quantity of solute is less than the solvent in a solution. the quantity of solvent is more than the solute in a solution. solute might exist in a solid, liquid, or gaseous state.

Solute Vs Solvent Definition 10 Major Differences And Examples The A solute is a molecule or particle that is distributed in a solvent. there will always be less solute than solvent. that means in a solution the solute is always the minor component. typically, the solute will be uniformly distributed in the solvent after mixing. practically, the solute is also usually being added to the solvent. The solute is the dispersed phase of a solution. the solvent is the medium phase of a solution that disperses solute particles. the quantity of solute is less than the solvent in a solution. the quantity of solvent is more than the solute in a solution. solute might exist in a solid, liquid, or gaseous state. Solute and solvent are two essential components of a solution. the solute refers to the substance that is dissolved in a solution, while the solvent is the substance that dissolves the solute. the solute is typically present in a smaller quantity compared to the solvent. it can be a solid, liquid, or gas, depending on the nature of the solution. The solutions that are exemplified in exercise 7.2.1 7.2. 1 contain solid, liquid, and gaseous solutes and solvents. as shown below in table 7.2.1 7.2. 1, solutions can be prepared using solvents and solutes in any state of matter combination, and the physical form of the resultant solution corresponds to the phase of its constituent solvent.

Difference Between Solvent And Solute Compare The Difference Betwee Solute and solvent are two essential components of a solution. the solute refers to the substance that is dissolved in a solution, while the solvent is the substance that dissolves the solute. the solute is typically present in a smaller quantity compared to the solvent. it can be a solid, liquid, or gas, depending on the nature of the solution. The solutions that are exemplified in exercise 7.2.1 7.2. 1 contain solid, liquid, and gaseous solutes and solvents. as shown below in table 7.2.1 7.2. 1, solutions can be prepared using solvents and solutes in any state of matter combination, and the physical form of the resultant solution corresponds to the phase of its constituent solvent.

Comments are closed.