Solved Calculate The Ph Of 1 00 L Of A Buffer That Is 0 10
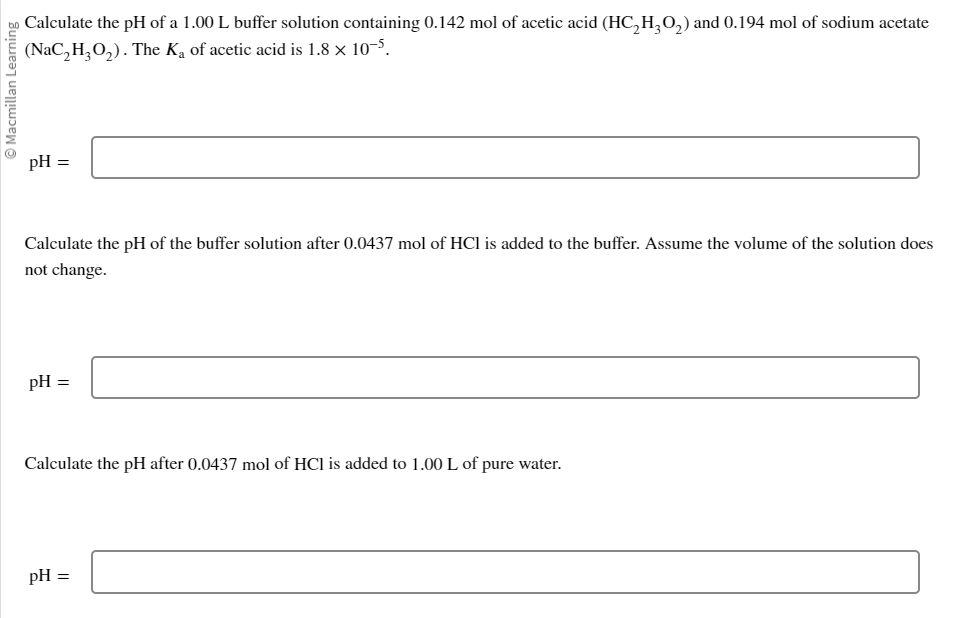
Solved Calculate The Ph Of A 1 00 L Buffer Solution Chegg Chemical education digital library (chemed dl) let us now consider the general problem of finding the ph of a buffer solution which is a mixture of a weak acid ha, of stoichiometric concentration c a, and its conjugate base a –, of stoichiom. [h3o ] = ka × [ha] [a−] (7.24.1) (7.24.1) [h 3 o ] = k a × [ha] [a −] taking negative. Calculate the ph of the solution after the addition of .150 moles of solid lioh. assume no volume change upon the addition of base. the ka for hf is 3.5*10^ 4. 2) a 1.00 l buffer solution is 0.250 m in hf and 0.250 m in naf. calculate the ph of the solution after the addition of 0.050 moles of solid naoh. assume no volume change upon the.

Solved Calculate The Ph Of 1 00 L Of The Buffer Chegg How do you calculate the ph of the solution after the addition of 100.0 ml of 1.00 m #hcl#? the ka for #hc 7h 5o 2# is #6.5 × 10^–5#. chemistry reactions in solution buffer calculations. A 1.00 l buffer solution is 0.250 m in hf and 0.250 m in naf. calculate the ph of the solution after the addition of 100.0 ml of 1.00 m hcl. (ka = 3.5 x 10^ 4) (a) 4.11 (b) 3.82 (c) 3.46 (d) 3.09. Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. to decide whether your buffer is based on acid & its conjugate base or the other way around, enter the data, and let the calculator do the job for you. in the article below, we will instruct you on how to calculate the ph of a buffer. A ph buffer solution is an aqueous solution capable of maintaining its ph stable against the addition of small quantities of strong acids and bases this behavior is of fundamental importance in biological systems, where an ideal range of ph is often required to allow certain processes.

Solved Calculate The Ph Of A 1 00 L Buffer Solution Chegg Our buffer ph calculator provides you with an effortless way to compute the ph of any kind of buffer solution. to decide whether your buffer is based on acid & its conjugate base or the other way around, enter the data, and let the calculator do the job for you. in the article below, we will instruct you on how to calculate the ph of a buffer. A ph buffer solution is an aqueous solution capable of maintaining its ph stable against the addition of small quantities of strong acids and bases this behavior is of fundamental importance in biological systems, where an ideal range of ph is often required to allow certain processes. Example #4: (a) calculate the ph of a 0.500 l buffer solution composed of 0.700 m formic acid (hcooh, k a = 1.77 x 10¯ 4) and 0.500 m sodium formate (hcoona). (b) calculate the ph after adding 50.0 ml of a 1.00 m naoh solution. solution to (a): we can use the given molarities in the henderson hasselbalch equation: ph = pk a log [base acid]. How to calculate the ph of a buffer solution using the henderson hasselbalch equation. watch a video tutorial with examples and practice problems.

Comments are closed.