Solved For A Ternary Phase Diagram Shown Below Write The Chegg
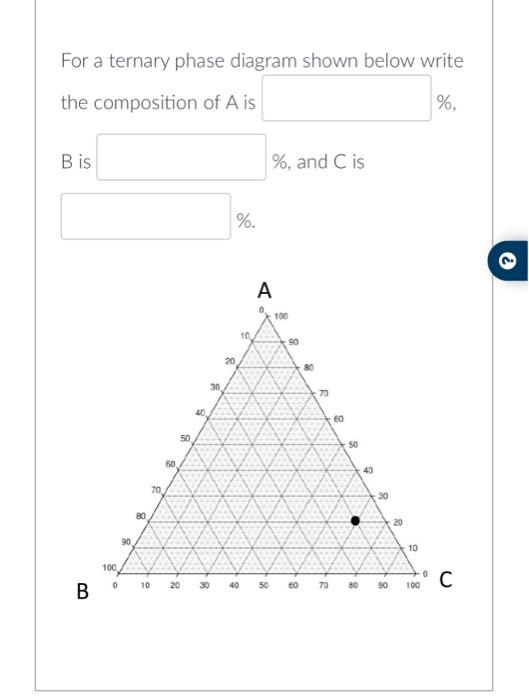
Solved For A Ternary Phase Diagram Shown Below Write The Chegg For a ternary phase diagram shown below write the composition of a is %, b is %, and c is %. this problem has been solved! you'll get a detailed solution from a subject matter expert that helps you learn core concepts. One component phase diagram. figure 1 illustrates the temperatures and pressures at which water can exist as a solid, liquid or vapor. the curves represent the points at which two of the phases coexist in equilibrium. at the point tt vapor, liquid and solid coexist in equilibrium. in the fields of the diagram (phase fields) only one phase exists.
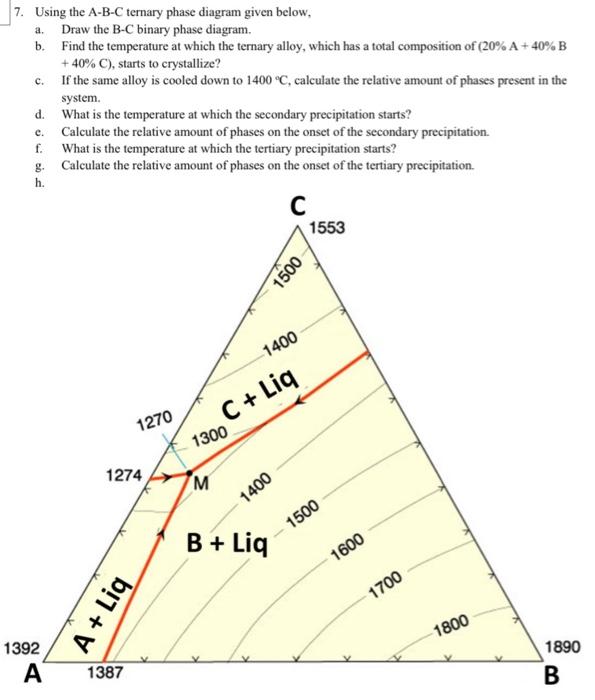
Solved 7 Using The A B C Ternary Phase Diagram Given Below Chegg T = 650 ° liq c a b. t < 650 ° c a b (all solid) at any temperature an isothermal plane can be constructed through the system that will show the phases present for all compositions in the ternary system. such an isothermal plane for the system abc at 700 ° is shown in figure 4. ii. 13.2.2 solid–liquid systems. figure 13.1 temperature–composition phase diagram for a binary system exhibiting a eutectic point. figure 13.1 is a temperature–composition phase diagram at a fixed pressure. the composition variable is the mole fraction of component b in the system as a whole. A typical phase diagram for such a mixture is shown in figure 8.6.2 8.6. 2. some combinations of substances show both an upper and lower critical temperature, forming two phase liquid systems at temperatures between these two temperatures. an example of a combination of substances that demonstrate the behavior is nicotine and water. Phase diagram and “degrees of freedom”. phase diagrams is a type of graph used to show the equilibrium conditions between the thermodynamically distinct phases; or to show what phases are present in the material system at various t, p, and compositions. “equilibrium” is important: phase diagrams are determined by using slow cooling.

Comments are closed.