Solved The Phase Diagram For The Ternary System Nh4cl Chegg
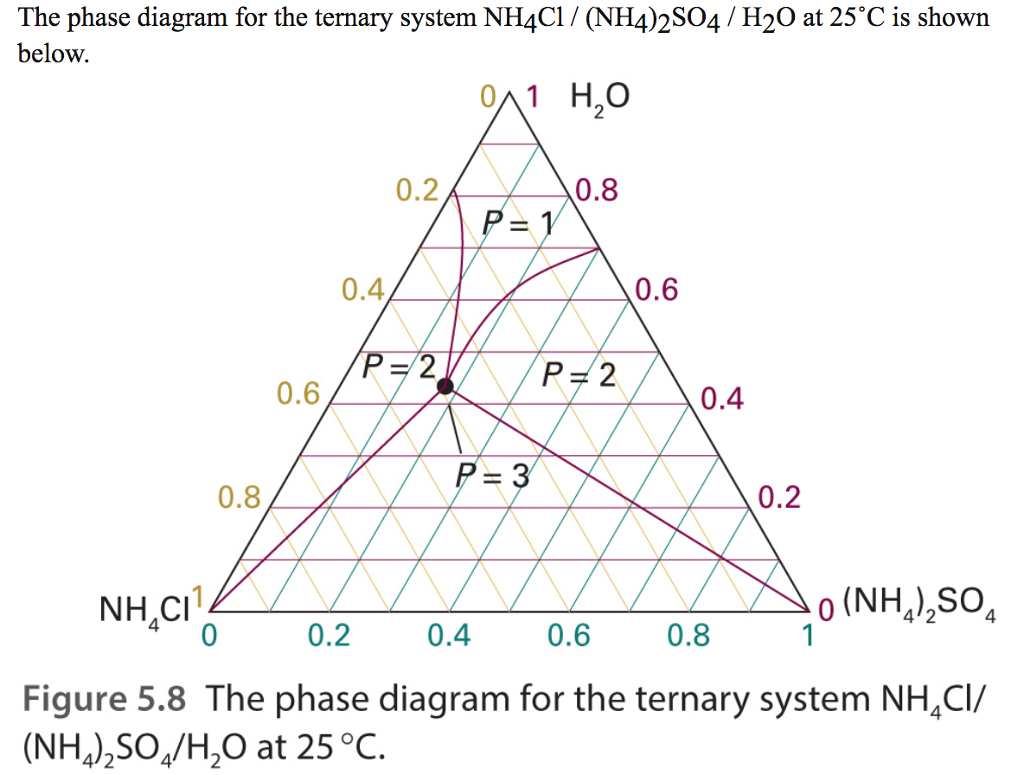
Solved The Phase Diagram For The Ternary System Nh4cl Chegg Question: the phase diagram for the ternary system nh4cl (nh4)2so4 h2o at 25˚c is shown in part 5. identify the number of phases present for mixtures of composition: i) (0.4, 0.1, 0.5) ii) (0.8, 0.1, 0.1) iii) (0, 0.3, 0.7) the phase diagram for the ternary system nh 4 cl (nh 4) 2 so 4 h 2 o at 25˚c is shown in part 5. identify the. The numbers are molefractions of the three components in the order: (nh4cl, (nh4)2so4, h2o). the phase diagram for the ternary system nh4cl (nh4)2so4 h2o at 25c is shown. identify the number of phases present for mixtures of composition.

Solved 4 Using The Phase Diagram Below For The Ternary Chegg Note that the relationship among the concentrations of the components is more complex than that of binary systems. figure 5.4.1 5.4. 1: three component triangular representation (i.e. 'blank graph paper' for a ternary diagram phase diagram), with axis labels perpendicular to each plotting axis to facilitate plotting and comprehension. The following data are given for the system nh4cl nh4no3 h2o at 25°c. construct the phase diagrams for the system and determine the ternary composition. is there evidence of hydrate or double salt formation? determine the maximum theoretical recovery of nh4cl from a dry mixture of nh4cl nh4no3 containing 80% by weight of nh4cl. Example problem 2. a pure solvent (flow rate= 299.4 kg h) is used to extract a solute from a feed (flow rate= 50.0 kg h) that is 35% solute and 65% carrier. what are the compositions and flow rates of the extract (the solvent rich phase leaving the stage) and of the raffinate (the effluent carrier phase from which solute was extracted) streams?. A partial solubility phase diagram of the ternary system was predicted within the temperature range of 273.15–323.15 k. it was found that based on the predicted solubility phase diagram there are two ternary peritectic points at temperatures below 300 k with one peritectic temperature at 299.65 k and the other at 298.15 k.
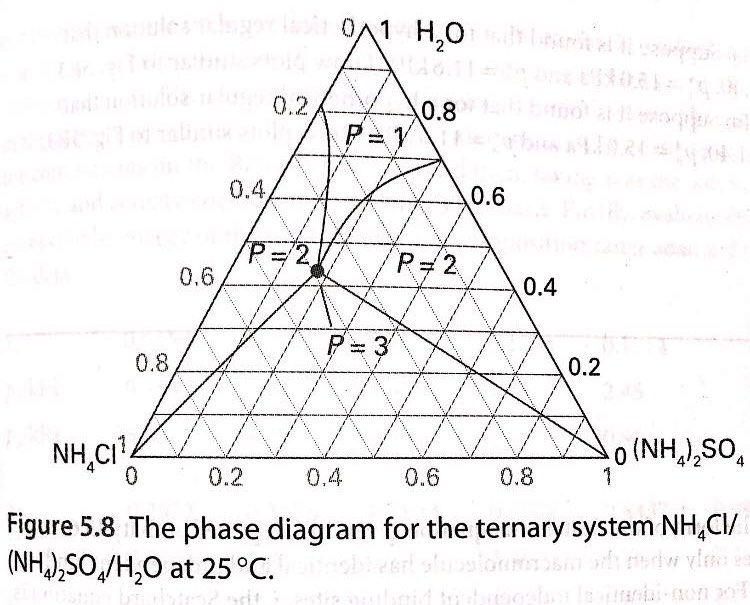
Solved The Phase Diagram For The Ternary System Chegg Example problem 2. a pure solvent (flow rate= 299.4 kg h) is used to extract a solute from a feed (flow rate= 50.0 kg h) that is 35% solute and 65% carrier. what are the compositions and flow rates of the extract (the solvent rich phase leaving the stage) and of the raffinate (the effluent carrier phase from which solute was extracted) streams?. A partial solubility phase diagram of the ternary system was predicted within the temperature range of 273.15–323.15 k. it was found that based on the predicted solubility phase diagram there are two ternary peritectic points at temperatures below 300 k with one peritectic temperature at 299.65 k and the other at 298.15 k. Solubility isotherms in the ternary system nh 4 cl cacl 2 h 2 o at t = (273.15 and 298.15) k were determined by the isothermal method. it was found that there are two solubility branches for cacl 2 ⋅6h 2 o and nh 4 cl at both temperatures. a pitzer–simonson–clegg model was selected to represent thermodynamic properties of this system. The kcl–nh4cl–h2o system is common in the potash industry, but it is difficult to obtain pure kcl in this system because of the mixed crystals of kcl and nh4cl. therefore, phase diagrams of.

Solved 4 Ternary Phase Diagram The Followings Are The Phase Cheggођ Solubility isotherms in the ternary system nh 4 cl cacl 2 h 2 o at t = (273.15 and 298.15) k were determined by the isothermal method. it was found that there are two solubility branches for cacl 2 ⋅6h 2 o and nh 4 cl at both temperatures. a pitzer–simonson–clegg model was selected to represent thermodynamic properties of this system. The kcl–nh4cl–h2o system is common in the potash industry, but it is difficult to obtain pure kcl in this system because of the mixed crystals of kcl and nh4cl. therefore, phase diagrams of.
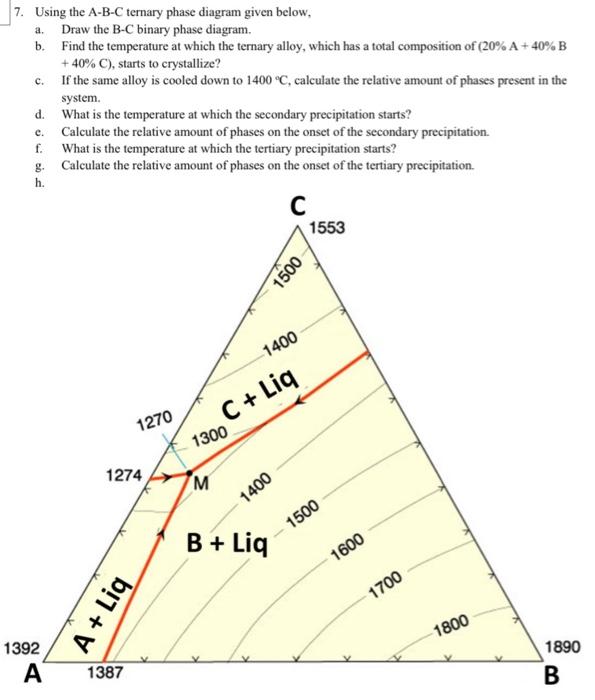
Solved 7 Using The A B C Ternary Phase Diagram Given Below Chegg

Comments are closed.