Solvent Examples List Types Uses

Solvent Examples List Types Uses Examples: chloroform has a density of 1.49 g cm³. d) low density type: these liquids have low density due to which their weight per volume is lower. examples: petroleum ether has a density of 0.64 g cm 3. examples of solvents used at home. from the above list you might find that few of them could be found at home on regular basis. the solvents. Water is a polar, protic solvent with the chemical formula h 2 o. water has the ability to dissolve a large variety of substances. this is the reason why it is regarded as a good solvent. furthermore, water is often referred to as the “universal solvent” because it is known to dissolve more substances than all other liquid solvents.
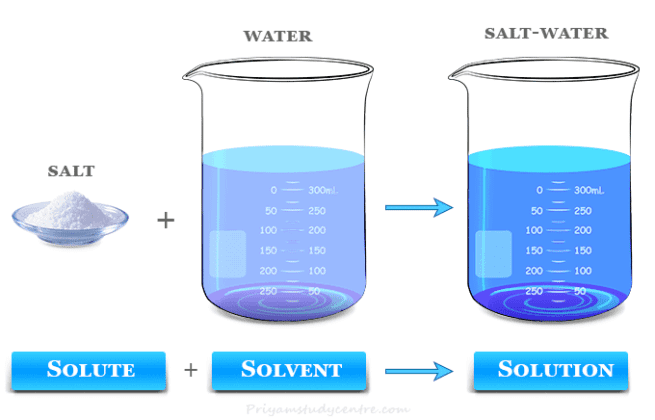
Solvent Definition Types Examples Chemistry Methanol, a versatile compound widely used as a laboratory solvent, offers a multitude of properties and applications. with its colorless and volatile nature, methanol has a molar mass of 32.04 g mol and a low molecular density. it exhibits excellent solubility in water and reaches a boiling point of 148.46°f (64.70°c). Polar protic solvent. a polar protic molecule is made up of an oh polar group with a non polar tail. a formula r oh can be used to represent the structure. they dissolve other polar protic molecular compounds. they are water miscible (hydrophilic). water (h oh), acetic acid (ch 3 co oh), and methanol (ch 3 oh) are examples of polar protic solvents. Oxygenated solvents. these solvents contain oxygen in their molecular structure and can be further classified into alcohols, ketones, esters, ethers, and glycol ethers. alcohols: methanol, ethanol, isopropanol, and butanol are examples of alcohol solvents. alcohols exhibit excellent solvency for various substances, making them versatile. Dry cleaning, paint thinners, nail polish removers, glue solvents, spot removers, detergents, and scents are a few applications of solvents. polar molecules can be dissolved in water. water dissolves practically all solutes, making it the most used solvent. proteins and ions found inside a live cell dissolve in water as well.

Solvent Oxygenated solvents. these solvents contain oxygen in their molecular structure and can be further classified into alcohols, ketones, esters, ethers, and glycol ethers. alcohols: methanol, ethanol, isopropanol, and butanol are examples of alcohol solvents. alcohols exhibit excellent solvency for various substances, making them versatile. Dry cleaning, paint thinners, nail polish removers, glue solvents, spot removers, detergents, and scents are a few applications of solvents. polar molecules can be dissolved in water. water dissolves practically all solutes, making it the most used solvent. proteins and ions found inside a live cell dissolve in water as well. 7. solvents in paints. 8. solvents in aerosols. 1. water (the “universal solvent”) we all are familiar with the fact that water is one of the most essential elements for any known lifeform to exist. in fact, water makes up approximately 62% of the human body and around three fourth of the surface of the earth. Some of the uses of solvents are dry cleaning, paint thinners, nail polish removers, glue solvents, spot removers, detergents, and perfumes. water is a solvent for polar molecules. water is the most common solvent as it has the capacity to dissolve almost all solute. ions and proteins present in a living cell also dissolve in water inside a cell.

Solvent Definition Types Incredible Uses Examples 7. solvents in paints. 8. solvents in aerosols. 1. water (the “universal solvent”) we all are familiar with the fact that water is one of the most essential elements for any known lifeform to exist. in fact, water makes up approximately 62% of the human body and around three fourth of the surface of the earth. Some of the uses of solvents are dry cleaning, paint thinners, nail polish removers, glue solvents, spot removers, detergents, and perfumes. water is a solvent for polar molecules. water is the most common solvent as it has the capacity to dissolve almost all solute. ions and proteins present in a living cell also dissolve in water inside a cell.

Comments are closed.