Structure And Classification Of Car T Cells Car T Cells Ar

Structure And Classification Of Car T Cells Car T Cells Car t cells are grouped into different generations depending on the structure of the car. recent advancements have added new car structures, which are extensively reviewed in [5, 8]. scfv single. Abstract. chimeric antigen receptor (car) t cell therapy represents a major advancement in personalized cancer treatment. in this strategy, a patient's own t cells are genetically engineered to express a synthetic receptor that binds a tumor antigen. car t cells are then expanded for clinical use and infused back into the patient's body to.
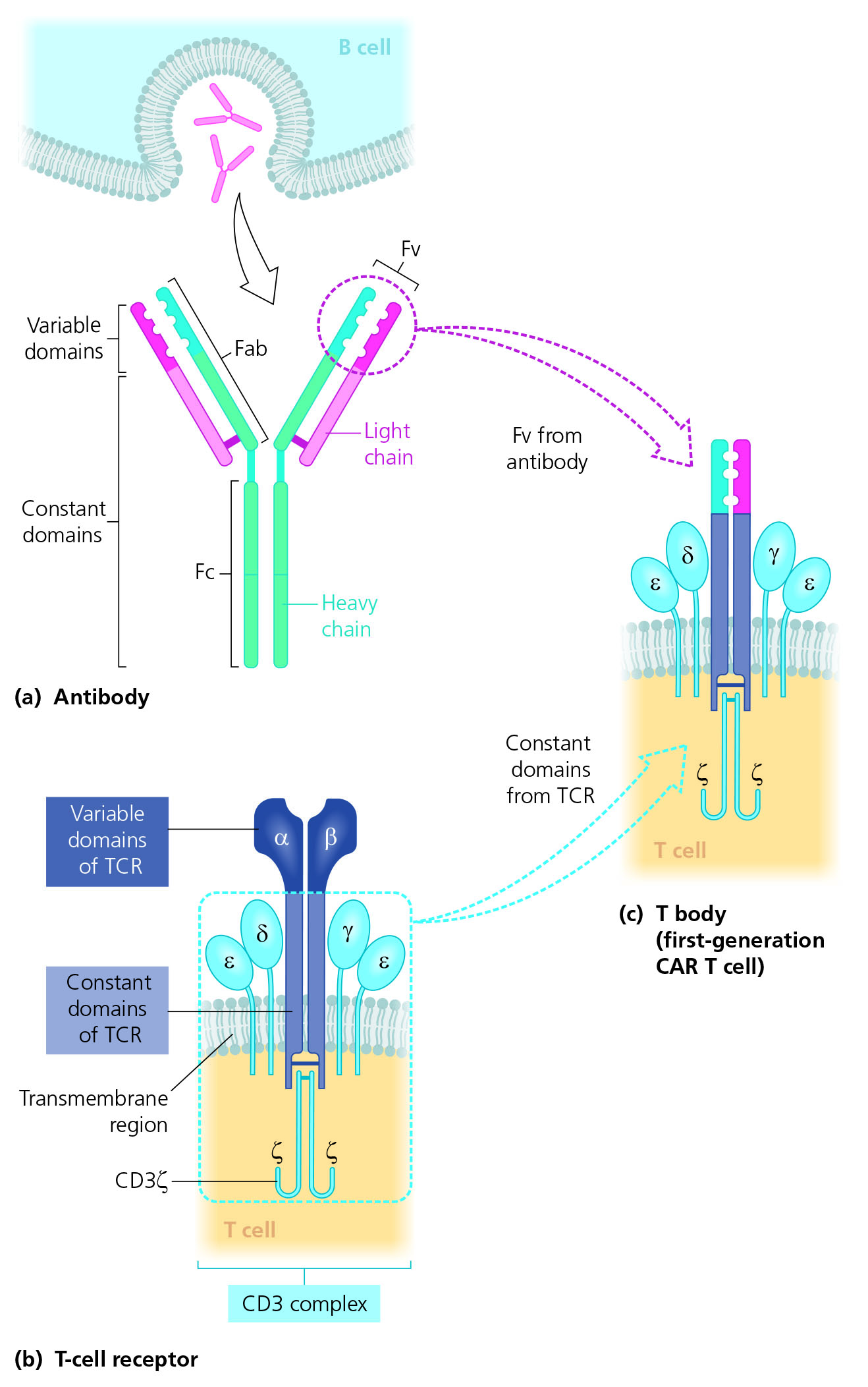
Structure Of Car T Cells вђ Leukaemia Care E Learning T cells genetically targeted with a chimeric antigen receptor (car) to b cell malignancies have demonstrated tremendous clinical outcomes. with the proof in principle for car t cells as a therapy for b cell malignancies being established, current and future research is being focused on adapting car technology to other cancers, as well as enhancing its efficacy and or safety. Chimeric antigen receptor (car) t cells use re engineered cell surface receptors to specifically bind to and lyse oncogenic cells. two clinically approved car t–cell therapies have significant clinical efficacy in treating cd19 positive b cell cancers. with widespread interest to deploy this immunotherapy to other cancers, there has been great research activity to design new car structures. The car is a chimeric transmembrane receptor with an extra membrane domain, responsible for antigen cd19 recognition via a single chain variable fragment structure. it is linked through a transmembrane domain to an intracellular component responsible for the t cell activation against its target. commercial car t cells are obtained from. Chimeric antigen receptor (car) t cell therapy is a promising form of immunotherapy that has seen significant advancements in the past few decades. it involves genetically modifying t cells to target cancer cells expressing specific antigens, providing a novel approach to treating various types of cancer. however, the initial success of first generation car t cells was limited due to.
Chimeric Antigen Receptor T Cell Structure Car T Indicates Chimeric The car is a chimeric transmembrane receptor with an extra membrane domain, responsible for antigen cd19 recognition via a single chain variable fragment structure. it is linked through a transmembrane domain to an intracellular component responsible for the t cell activation against its target. commercial car t cells are obtained from. Chimeric antigen receptor (car) t cell therapy is a promising form of immunotherapy that has seen significant advancements in the past few decades. it involves genetically modifying t cells to target cancer cells expressing specific antigens, providing a novel approach to treating various types of cancer. however, the initial success of first generation car t cells was limited due to. The advent of chimeric antigen receptor (car) t cell has changed the treatment landscape for hematologic malignancies, offering substantial benefits in terms of response rates for diseases such as leukemia, 1, 2 lymphoma, 3, 4 and multiple myeloma. 5, 6 however, the response rates of car t were not as satisfactory in solid tumors, 7, 8 attributable to various interwind factors such as minimal. Introduction. chimeric antigen receptor (car) t cell therapy, in which a patient’s own t lymphocytes are engineered to recognize and kill cancer cells, has achieved remarkable success in some hematological malignancies in preclinical and clinical trials, resulting in six fda approved car t products currently available in the market [1–6] (table 1) (fig. 1).

Comments are closed.