Sublimating Dry Ice
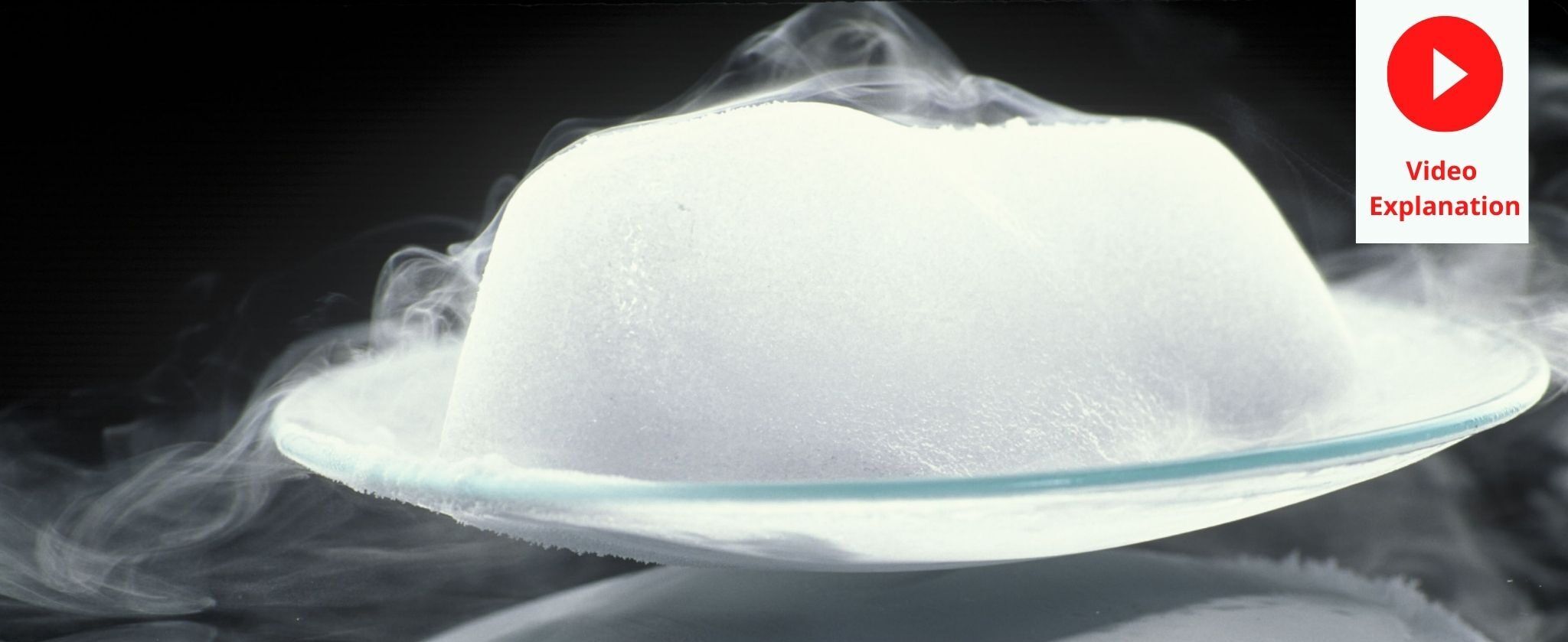
Sublimating Dry Ice Copyright 2017 turtle rock scientificthis video can be licensed at sciencesource archive dry ice sublimating ss2857338 dry ice is solid c. A container holding dry ice (frozen carbon dioxide) sublimating into the air. "dry ice" is actually solid, frozen carbon dioxide, which happens to sublimate, or turn to gas, at a chilly 78.5 °c ( 109.3°f).

Dry Ice Sublimating Youtube This is a basic introductory demonstration to the nature of dry ice (solid carbon dioxide), which sublimes (goes straight from being a solid to a gas) under. Through demonstrations with dry ice (the solid state of carbon dioxide), the students were able to observe one particularly exciting transition in action: sublimation (solid to gas). we studied sublimation by adding dry ice to water. the warmth of the water causes the dry ice to sublimate rapidly, releasing clouds of white vapor (carbon dioxide. Sublimation (phase transition) sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. [1] the verb form of sublimation is sublime, or less preferably, sublimate. [2] sublimate also refers to the product obtained by sublimation. [2][3] the point at which sublimation occurs. Dry ice is solid. it sublimates or changes states from a solid to a gas at temperatures of 78 degrees celsius under normal atmospheric pressure of 1 atm. because of its low temperature at normal atmospheric pressure, it is useful as a coolant. when dry ice is placed into warm water, a cloud forms. this cloud is similar to the clouds we see in.

Dry Ice Sublimating Stock Image C039 1177 Science Photo Library Sublimation (phase transition) sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. [1] the verb form of sublimation is sublime, or less preferably, sublimate. [2] sublimate also refers to the product obtained by sublimation. [2][3] the point at which sublimation occurs. Dry ice is solid. it sublimates or changes states from a solid to a gas at temperatures of 78 degrees celsius under normal atmospheric pressure of 1 atm. because of its low temperature at normal atmospheric pressure, it is useful as a coolant. when dry ice is placed into warm water, a cloud forms. this cloud is similar to the clouds we see in. 1. place the dry ice in the bag. 2. remove excess air and seal the bag. 3. observe the dry ice sublimating and the co 2 gas expanding within the bag. 4. place a book or other object on the bag to emphasize the work being done by the gas on the surroundings. Procedure. place a piece of dry ice in a balloon and tie the balloon. as the dry ice sublimes the balloon inflates. pass the balloon around the classroom. or you could place several pieces of dry ice into an erlenmeyer flask and then attach the balloon over the mouth of the flask. this balloon will inflate as the dry ice sublimes.

Sublimating Dry Ice 1. place the dry ice in the bag. 2. remove excess air and seal the bag. 3. observe the dry ice sublimating and the co 2 gas expanding within the bag. 4. place a book or other object on the bag to emphasize the work being done by the gas on the surroundings. Procedure. place a piece of dry ice in a balloon and tie the balloon. as the dry ice sublimes the balloon inflates. pass the balloon around the classroom. or you could place several pieces of dry ice into an erlenmeyer flask and then attach the balloon over the mouth of the flask. this balloon will inflate as the dry ice sublimes.

Comments are closed.