Sublimation Examples In Daily Life 5 Examples Teachoo Science

Sublimation Examples In Daily Life 5 Examples Teachoo Science 10 examples of sublimation. last updated at april 16, 2024 by teachoo. dry ice is the solid form of carbon dioxide. when dry ice is placed in contact with air, it gets converted into the gaseous carbon dioxide which appears like fog. ice and snow under certain conditions also show the presence of gases around the solid as the ice or snow sublimes. Ans: sublimation, including melting, freezing, and evaporation, is a form of phase transition, or shift in a state of matter. a substance transforms from a solid to a gas by sublimation without ever going through a liquid phase. a common example of sublimation is the dry ice, heavy co2. 2. how is sublimation used in everyday life?.
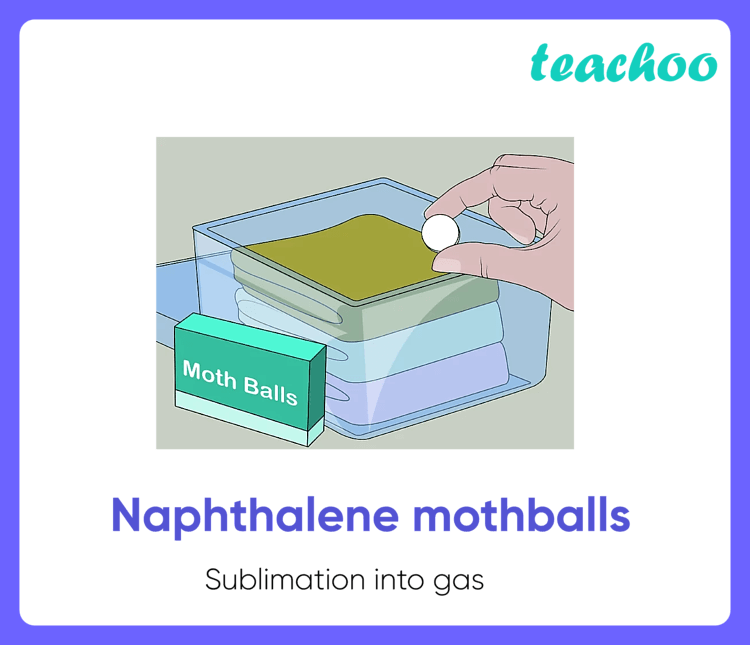
Sublimation Examples In Daily Life 5 Examples Teachoo Science Sublimation has several practical applications across various fields. here are some examples: [2,5,6] 1. freeze drying (lyophilization): sublimation is used in freeze drying to remove moisture from substances such as food, pharmaceuticals, and biological samples while preserving their structure and properties. Similarly, the combustion of candles containing paraffin wax into carbon dioxide and water vapor is also not sublimation, but a chemical reaction with oxygen. in general, sublimation only attributes to the change of the physical state of the matter. let’s discuss a few examples of sublimation in daily life: 1. dry ice. Pharmaceutical tablets. 22. freeze dried coffee. here are a few examples of sublimation in daily life: 1. dry ice. dry ice is the solid form of carbon dioxide. it sublimates at atmospheric pressure. this means that it changes directly from a solid to a gas without melting into a liquid. Examples of sublimation carbon dioxide. solid carbon dioxide (dry ice) sublimes everywhere along the line below the triple point at pressures and temperatures above the triple point (for example, at 5.1 atmospheres and 56.6 degrees celsius, for a temperature of 194.65 degrees kelvin, or 109.30 degrees fahrenheit), whereas its melting into liquid co 2 can occur anywhere along the solid liquid.

Comments are closed.